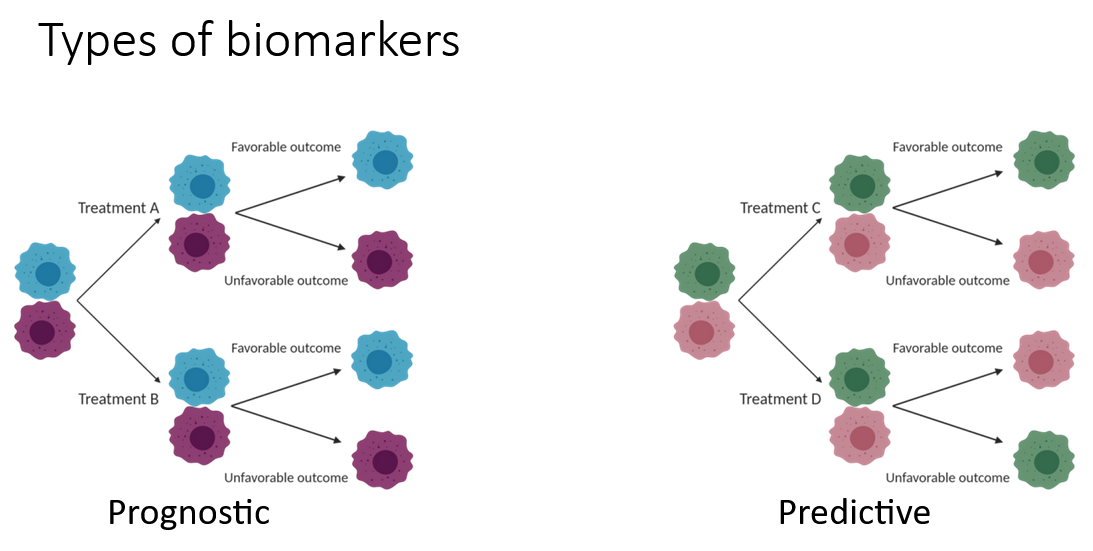
Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 3: Immune Checkpoint Blockade, will cover the biological foundation of immune checkpoint blockade therapies as well as diving into their clinical use.
- Original Release Date: December 1, 2022
- Expiration Date: January 8, 2026
- Date of Last Review: January 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Click on image for FREE PREVIEW
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic | At the conclusion of this activity, participant should be able to: |
| Basic Mechanisms of Checkpoint Blockade in Immunotherapy |
|
| Biomarkers of Response to ICB and General Clinical Utility |
|
| Immune Checkpoint Inhibitors and Combination Approaches |
|
| Monitoring Response to Checkpoint Blockade |
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty | Conflict of Interest |
|
Robert L. Ferris, MD, PhD Hillman Professor of Oncology Director, UPMC Hillman Cancer Center Associate Vice Chancellor for Cancer Research University of Pittsburgh Medical Center, Pittsburgh, PAnderson Cancer Center, Houston, TX |
|
| Certificate Program Task Force | Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair) |
|
|
Umar Farooq, MD |
|
|
Silvia Formenti, MD |
|
|
Sigrun Hallmeyer, MD |
|
|
Jose Lutzky, MD, FACP |
|
|
George Weiner, MD |
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-014-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 1/8/2024 through 1/8/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.