|
|
SITC connectED Researcher Portal
Browse online courses and resources for researchers by cancer type, general immunotherapy courses, or click All Researcher Courses to view all available courses. Click the Additional Researcher Resources button to explore more SITC resources for researchers.
|
 | Published: On 08/11/2023
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
The course- Combination therapies in Combating Cancer gives a brief overview of the several combination therapies such as Inhibitory Immune Checkpoint Combination therapy, Targeted Immunotherapy approches, and other emerging combination therapies. In this course, the subject matter expert describes the effectiveness of various combination immunotherpy approches and their outcomes in cancer patients using several case studies and research papers. The course content emphasizes the emerging landscape of combining immunotherapy with different modalities to treat cancer.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete activity: 1 hour
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe key immunotherapy targets that are useful for combination approaches.
- Describe immunotherapy-based combination treatment regimens currently under investigation.
- Discuss the emerging landscape of combining immunotherapy with different modalities to treat cancer.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty
Gregory B. Lesinski, PhD, MPH
Co-Director, Translational GI Malignancy Program
Winship Cancer Institute of Emory University,
Atlanta, Georgia, USA
Disclosure of Conflicts of Interest
The faculty reported the following relevant financial relationships with ineligible entities related to the education content of this CE activity:
Gregory B. Lesinski, PhD, MPH: Works at a Academic Medical Center and a consultant advisor speaker for Bristol-Myers Squib, Vaccinex, Boerhinger-Ingelheim, Merck and Co.
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: August 11, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Please note: this course is no longer eligible for continuing education credits.
Published December 24, 2020
Course Description
This interactive course provides an overview of the mechanisms by which cancer immunotherapy may activate immune-related adverse events (irAEs). The foundational knowledge presented in this course is critical to understanding how and why immune-related adverse events occur and how they can be treated.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Pradnya Patil, MD, FACP
Hematology/Oncology Fellow
Taussig Cancer Institute, Cleveland Clinic
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Discuss the mechanisms of peripheral and central immune tolerance.
- Describe the biological pathways involved in mediating autoimmune reactions.
- Explain the differences between PD-1 and CTLA-4 pathway-mediated autoimmunity.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: December 24, 2020 - December 24, 2021
Estimated time to complete activity: 0.5 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2020-2021 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb, Exelixis, Inc., and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2520-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Pradnya Patil, MD, FACP: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: December 24, 2020
|
Approved Credit: ACPE: 0.50 hours Contact HourANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version, go here to find the What's Next in Cancer Immunotherapy that is a CME-, CPE-, CNE-, and MOC-certified activity.
Published February 11, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, What's Next for Cancer Immunotherapy?, covers the future of cancer immunotherapy such as understanding important trends, new developments, potential approvals and disease states with promising data.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Evan J. Lipson, MD
Associate Professor, Department of Oncology
Johns Hopkins University School of Medicine
Parul Agarwal, MD
Medical Oncology Fellow
Johns Hopkins University School of Medicine
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale behind expanding the settings in which cancer immunotherapy is administered, including patients with a larger variety of tumor types and patient populations previously excluded from clinical trial participation.
- Describe the basis for novel immunotherapy drug combinations.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 2/11/20 - 2/11/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-1083-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.4 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Evan J. Lipson, MD: Consultant (Bristol-Myers Squibb, Novartis, EMD Serono, Array BioPharma, Macrogenics, Merck); Contracted Research (Bristol-Myers Squibb, Merck)
Parul Agarwal, MD: Nothing to Disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 2/11/20 through 2/11/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for What's Next for Cancer Immunotherapy?
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: February 11, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version go here to find the Mechanisms of Immune-Related Adverse Events that is a CME-, CPE-, CNE- and MOC-certified activity.
Published December 23, 2019
Course Description
This interactive course provides an overview of the mechanisms by which cancer immunotherapy may activate immune-related adverse events (irAEs). The foundational knowledge presented in this course is critical to understanding how and why immune-related adverse events occur and how they can be treated.
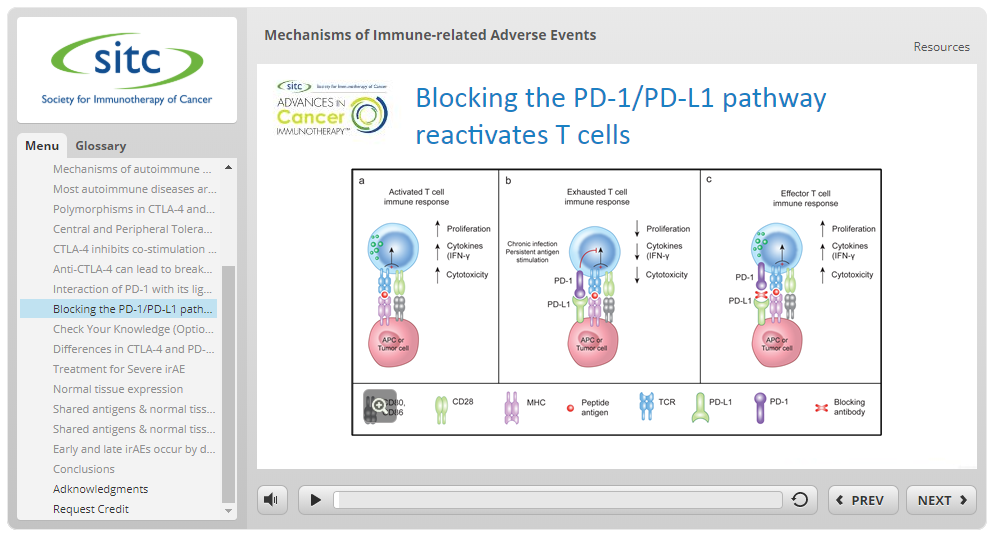
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Pradnya Patil, MD, FACP
Hematology/Oncology Fellow
Taussig Cancer Institute, Cleveland Clinic
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Discuss the mechanisms of peripheral and central immune tolerance.
- Describe the biological pathways involved in mediating autoimmune reactions.
- Explain the differences between PD-1 and CTLA-4 pathway-mediated autoimmunity.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: December 23, 2019 - December 23, 2020
Estimated time to complete activity: 0.5 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-887-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Pradnya Patil, MD, FACP: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period December 23, 2019 through December 23, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for "Mechanisms of Immune-Related Adverse Events."
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete the post-test with a passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: December 23, 2019
|
Approved Credit: ACPE: 0.50 hours Contact HourANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Published December 17, 2019
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This Basic Principles of Cancer Immunotherapy course covers the basic principles and mechanisms behind cancer immunotherapy.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses, and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Stefani Spranger, PhD
Assistant Professor of Biology
Koch Institute for Integrative Cancer Research at MIT
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy
-
Recognize various approaches to cancer immunotherapy including therapeutic vaccine, adoptive cell transfer, cytokines, checkpoint inhibitor modulation, effector antibodies and antibody-drug conjugates
-
Explain the mechanism by which tumors evade the immune system and mechanisms to overcome this immunosuppression
-
List important biomarkers for measuring the immune response
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 12/17/19-12/17/20
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-991-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.3 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Stefani Spranger, PhD: Consulting fees to Venn Therapeutic, Tango, Takeda, Replimune, Ribon, Dragonfly, Merck, Torque; Contracted Research to Takeda, Exelixis
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 12/17/19 through 12/17/20 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Basic Principles of Cancer Immunotherapy.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Firefox, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: December 17, 2019
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This course is no longer available for continuing education credits. Further, this course has been replaced by Module 1 of the SITC Certificate in Cancer Immunotherapy program, Basic Immunology Concepts, as serving as one of the pre-program courses for the ACI Live Virtual events. If you've registered for an ACI Live Virtual event you should have received a coupon code to take Basic Immunology Concepts for free.
Course Description
This interactive course provides an overview of the immune system, focusing on the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The foundational knowledge presented in this course is critical to understanding how the immune system can be harnessed to treat cancer through immunotherapy.

Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Christian M. Capitini, MD
Assistant Professor
University of Wisconsin Carbone Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the function of the components of the immune system, including relevant cells, molecules and organs of the immune system.
- Differentiate between the adaptive immune system and the innate immune system.
- Recognize the barriers to effective immunotherapy, including mechanisms by which tumors locally disable and/or evade the immune system.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: October 9, 2019 - October 9, 2020
Estimated time to complete activity: 1 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.0 contact hour (0.10 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-884-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.0 contact hour. Designated for 0.5 pharmacotherapy contact hour for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Christian Capitini, MD: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period October 9, 2019 through October 9, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for "Introduction to Immunology – Third Edition."
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: October 09, 2019
|
Approved Credit: ACPE: 1 hour Contact HourANCC: 1 hour Contact HourACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
|
|
|
|











 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.



