|
|
SITC connectED Clinician Portal
Browse online courses and resources for clinicians by date published, cancer type, or general immunotherapy courses. Click the Additional Clinician Resources button to explore more SITC resources for clinicians.
To view courses available for CE credit, click the CE courses on the left menu or click More Info next to a course title.
|
 | 
Target Audience and Goal Statement
This educational activity is intended for an audience of oncologists, gastroenterologists, surgeons, and other clinicians on the multidisciplinary team in the United States.
The goal of this activity is for learners to be better able to incorporate immunotherapy strategies into the management of patients with HCC.
Upon completion of this activity, participants will:
- Have increased knowledge regarding the clinical trial data associated with immunotherapy-based strategies for frontline treatment of advanced unresectable HCC.
- Have greater competence related to selecting the most appropriate immunotherapy-based strategies for first-line treatment of advanced unresectable HCC.
- Demonstrate greater confidence in their ability to differentiate the benefits of immunotherapy-based combination regimens for first-line treatment of advanced unresectable HCC.

Available Beginning: December 13, 2024
Approximate Time to Complete: 30 minutes
CE Credits: 0.50 AMA PRA Category 1 Credit™/ABIM MOC points; available through 12/13/2025, 11:59 PM EST
Developed through a partnership between SITC and Medscape.
| | Original Course Date: December 02, 2024
| MORE INFO |
 | .png)
Advances in immunotherapy are changing the treatment landscape for non-small cell lung cancer (NSCLC), with immune checkpoint inhibitors rapidly expanding the treatment landscape in early-stage and advanced or metastatic disease. However, more awareness is needed among clinicians to remain up to date with the latest guidelines that reflect advances and approvals of these therapies. A panel led by two expert thoracic oncologists will discuss the most recent management guidelines, the significance of selecting patients’ therapy based on PD-L1 expression, and patient-centered treatment strategies as related to special populations. The panel will also review strategies for immunotherapy before and after surgery, and management of immune-related adverse events. The patient voice will be emphasized with a pre-recorded patient vignette highlighting patient experience and challenges when having concomitant NSCLC and a chronic infection.

Program Learning Objectives:
- Review guideline updates on immunotherapy in cancer and the role of PD-L1 expression testing in guiding treatment
- Outline strategies to identify patients who can benefit from peri-adjuvant, adjuvant, or neoadjuvant treatment for NSCLC
- Evaluate the latest safety and efficacy data on ICIs for NSCLC
- Outline integration of ICIs into treatment plans and individualization of therapy for special patient populations
Faculty
Abdul Rafeh Naqash, MD
Assistant Professor of Medicine
Director of Immuno-oncology
OU Health Stephenson Cancer Center at the University of Oklahoma Health Sciences
Balazs Halmos, MD
Professor of Oncology
Montefiore Medical Center
Approximate Time to Complete: 60 minutes
Credit Available: November 25, 2024 - November 25, 2025
Developed through a collaboration between SITC and PlatformQ.
| | Original Course Date: November 25, 2024
|
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | 
Melanoma Cancer CPG Mini-Module – Treatment options for advanced melanoma
 The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education. The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Elizabeth Buchbinder, MD, of Dana-Farber Cancer Institute presents two case studies of advanced disease. The first case study addresses available options and current recommendations for treating patients with advanced melanoma without BRAF mutations. This a case study includes a panel discussion between Dr. Buchbinder, Tina Hieken, MD, of Mayo Clinic, and April Salama, MD, of the Duke Cancer Institute about first-line therapy for regionally limited disease. The second case study of a patient with extensive stage BRAFV600-mutated melanoma includes a discussion of the current recommendations for treating BRAF-mutated melanoma as well as data comparing combination immune checkpoint inhibitor therapy with targeted triplet therapy and their associated clinical benefits.
Run time: Approximately 13 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants, and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described
in this educational activity.
The Mini Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: February 05, 2024
On-Demand Release Date: Available Now | MORE INFO |
 | 
Melanoma CPG Mini-Module – Multidisciplinary care for resectable melanoma
 The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education. The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Tina Hieken, MD, of Mayo Clinic, presents a case study of a patient with resectable melanoma and addresses benefits associated with immune checkpoint blockade therapy in the neoadjuvant and adjuvant settings. Discussion between Dr. Hieken with panelists Elizabeth Buchbinder, MD, of Dana-Farber Cancer Institute and April Salama, MD, of the Duke Cancer Institute touches on considerations for selecting appropriate treatment for patients, unanswered questions regarding neoadjuvant treatment, the need for larger clinical trials addressing immunotherapy in the neoadjuvant setting, and the importance of multidisciplinary care when treating patients with resectable disease.
Run time: Approximately 11 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants, and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described
in this educational activity.
The Mini Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: February 05, 2024
On-Demand Release Date: Available Now | MORE INFO |
 | 
Melanoma Cancer CPG Mini-Module – Management of melanoma brain metastases
 The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education. The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
In this case study presentation, Elizabeth Buchbinder, MD, of Dana-Farber Cancer Institute and April Salama, MD, of the Duke Cancer Institute address the guidelines for the management of melanoma brain metastases. Dr. Buchbinder also outlines current options for front-line treatment of advanced disease and patient-specific considerations associated with each option. This mini-module ends with a discussion between Drs. Buchbinder and Salama about the potential benefits and unanswered questions surrounding adoptive cellular therapy for melanoma.
Run time: Approximately 10 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants, and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described
in this educational activity.
The Mini Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: February 05, 2024
On-Demand Release Date: Available Now | MORE INFO |
 | Published: On Date
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description and Target Audience
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, advanced practitioners, emergency physicians and all members of the cancer team, the ACI program focuses on how to educate your patients about receiving inpatient/outpatient cellular therapies; including examples of practical implementation provided
Estimated time to complete activity: 25 Minutes
Learning Objectives
At the conclusion of this activity, the participant should be able to:
-
Understand the patient journey and how to prepare a patient for these steps.
-
To be informed of the many time points that require patient education and reinforcement.
-
Develop an understanding about the varying pieces of education necessary for patient success.
-
How to properly prepare patients and their caregivers for outpatient CAR-T cell therapy.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty and Disclosure of Conflicts of Interest
No relevant financial conflicts.
Faculty
Meaghan Ekstrom, BA, BSN, MSN, FNP-BC
Massachusetts General Hospital
Boston, MA
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: December 25, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published: On Date
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description and Target Audience
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, advanced practitioners, emergency physicians and all members of the cancer team, the ACI program focuses on how to educate your patients about receiving inpatient/outpatient cellular therapies; including examples of practical implementation provided
Estimated time to complete activity: 25 Minutes
Learning Objectives
At the conclusion of this activity, the participant should be able to:
-
Understand the patient journey and how to prepare a patient for these steps.
-
To be informed of the many time points that require patient education and reinforcement.
-
Develop an understanding about the varying pieces of education necessary for patient success.
-
How to properly prepare patients and their caregivers for outpatient CAR-T cell therapy.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty and Disclosure of Conflicts of Interest
No relevant financial conflicts.
Faculty
Meaghan Ekstrom, BA, BSN, MSN, FNP-BC
Massachusetts General Hospital
Boston, MA
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: December 25, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | 
 Melanoma, v3.0 CPG Mini-Module 1– Multidisciplinary care for resectable melanoma Melanoma, v3.0 CPG Mini-Module 1– Multidisciplinary care for resectable melanoma
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, these on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Tina Hieken, MD, of Mayo Clinic, presents a case study of a patient with resectable melanoma and addresses benefits associated with immune checkpoint blockade therapy in the neoadjuvant and adjuvant settings. Discussion between Dr. Hieken with panelists Elizabeth Buchbinder, MD, of Dana-Farber Cancer Institute and April Salama, MD, of the Duke Cancer Institute touches on considerations for selecting appropriate treatment for patients, unanswered questions regarding neoadjuvant treatment, the need for larger clinical trials addressing immunotherapy in the neoadjuvant setting, and the importance of multidisciplinary care when treating patients with resectable disease.
Run time: Approximately 11 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: December 07, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | 
 Gastrointestinal Cancer CPG Mini-Module – Management of lower gastrointestinal tumors Gastrointestinal Cancer CPG Mini-Module – Management of lower gastrointestinal tumors
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, these on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Cathy Eng, MD, FACP, FASCO, of Vanderbilt-Ingram Cancer Center, addresses the guidelines in the context of tumors in the lower gastrointestinal tract by presenting a case study of multidisciplinary management of metastatic colorectal cancer. Dr. Eng also discusses important considerations when deciding between single-agent immune checkpoint blockade or combination therapy and the clinical data underlying these considerations.
Run time: Approximately 15 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: December 05, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | 
 Gastrointestinal Cancer CPG Mini-Module – Immunotherapy biomarkers in the gastrointestinal tract Gastrointestinal Cancer CPG Mini-Module – Immunotherapy biomarkers in the gastrointestinal tract
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, these on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Kristen K. Ciombor, MD, MSCI, of Vanderbilt Ingram Cancer Center, Vanderbilt University, presents an overview of tumor-agnostic biomarkers including MSI-H/dMMR, POLE/POLD1 mutations, and TMB and the caveats of these biomarkers in the context of gastrointestinal cancers. Data from key clinical studies of these biomarkers are also discussed.
Run time: Approximately 11 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: December 05, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | 
 Gastrointestinal Cancer CPG Mini-Module – Patient selection for immunotherapy for esophagogastric cancer Gastrointestinal Cancer CPG Mini-Module – Patient selection for immunotherapy for esophagogastric cancer
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, these on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Geoffrey Ku, MD, of Memorial Sloan Kettering Cancer Center presents two case studies of esophagogastric cancer, illustrating how biomarkers can provide guidance for treating a patient’s individual disease. Dr. Ku also discusses key first-line clinical studies underlying the guidelines and the need to identify patient subpopulations most likely to benefit from immunotherapy. The mini-module concludes with a discussion between Dr. Ku and moderator Ronan J. Kelly, MD, MBA, of Baylor University Medical Center on specific factors to consider when treating patients with PD-L1-positive esophagogastric tumors and the caveats of PD-L1 testing.
Run time: Approximately 20 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: December 05, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | Published: On 10/05/2023
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
The course irAES associated with Cellular Therapies and T-cell Engagers starts with an overview of cellular therapies and bisepcific antibodies. Dr. Loretta Nastoupil of UT MD Anderson Cancer Center has described the immune related acute and late adverse events associated with CAR T-cell therapies and potential approches to management of toxicities. The case studies presented by Dr. Nastoupil help decide the best possible action plan for managing toxicities associated with CAR T-cell therapies in patients.
Estimated time to complete activity: 40 Minutes
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Learning Objectives
At the conclusion of this activity, the participant should be able to:
-
Give a brief overview of the mechanism of action and common toxicities associated with cellular therapies and T cell engagers.
-
List the multiple approaches that should be followed post treatment.
-
Describe the immune related acute and late adverse events (toxicities) associated with CAR-T cell therapies.
-
Describe similar acute toxicities associated with T-cell engagers and potential different approaches to management.
-
Explain the action plan for the management of toxicities associated with CAR-T cell therapies.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty and Disclosure of Conflicts of Interest
Faculty
Loretta Nastoupil, MD
Associate Professor
UT MD Anderson Cancer Center
Texas, USA
The faculty reported the following financial relationships or relationships to products or devices they have with ineligible companies:
Conflicts of Interest
Researcher: BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Takeda
Consultant/ Advisor/ Speaker: Abbvie, Atara, BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech, Gilead/Kite, Janssen, Merck, Novartis, Takeda
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: October 06, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published: On 10/05/2023
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
The course irAES associated with Cellular Therapies and T-cell Engagers starts with an overview of cellular therapies and bisepcific antibodies. Dr. Loretta Nastoupil of UT MD Anderson Cancer Center has described the immune related acute and late adverse events associated with CAR T-cell therapies and potential approches to management of toxicities. The case studies presented by Dr. Nastoupil help decide the best possible action plan for managing toxicities associated with CAR T-cell therapies in patients.
Estimated time to complete activity: 40 Minutes
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Learning Objectives
At the conclusion of this activity, the participant should be able to:
-
Give a brief overview of the mechanism of action and common toxicities associated with cellular therapies and T cell engagers.
-
List the multiple approaches that should be followed post treatment.
-
Describe the immune related acute and late adverse events (toxicities) associated with CAR-T cell therapies.
-
Describe similar acute toxicities associated with T-cell engagers and potential different approaches to management.
-
Explain the action plan for the management of toxicities associated with CAR-T cell therapies.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty and Disclosure of Conflicts of Interest
Faculty
Loretta Nastoupil, MD
Associate Professor
UT MD Anderson Cancer Center
Texas, USA
The faculty reported the following financial relationships or relationships to products or devices they have with ineligible companies:
Conflicts of Interest
Researcher: BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Takeda
Consultant/ Advisor/ Speaker: Abbvie, Atara, BMS, Caribou Biosciences, Daiichi Sankyo, Epizyme, Genentech, Gilead/Kite, Janssen, Merck, Novartis, Takeda
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: October 06, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | 
 Gynecologic Cancer CPG Mini-Module – A Case of Vulvar Melanoma Gynecologic Cancer CPG Mini-Module – A Case of Vulvar Melanoma
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Jyoti Bajpai, MBBS, MD, DM, of Tata Memorial Centre, Mumbai, India, presents a case study of pembrolizumab for metastatic vulvar melanoma. This case study addresses the relevance of the CPG recommendations for managing rare conditions like vulvar melanoma, necessary considerations when selecting resource-appropriate therapies in real-world situations, and studies supporting the use of supportive care or abbreviated courses of therapy when the standard of care is not available. This Mini-Module concludes with a discussion between Dr. Bajpai and Kunle Odunsi, MD, PhD, of University of Chicago Medicine on the need for more global collaboration to make immunotherapy accessible and affordable for patients around the world.
Run time: Approximately 14 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: September 29, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | 
Gynecologic Cancer CPG Mini Module – Immunotherapy Biomarkers in the Gynecologic Tract: MMR, PD-L1, and TMB
 The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education. The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Anne M. Mills, MD, of the University of Virginia, provides a comprehensive overview of three biomarkers associated with immunotherapy for gynecologic cancers, specifically mismatch repair (MMR), PD-L1, and tumor mutation burden (TMB). Topics discussed include immunohistochemistry- and PCR-based testing for these biomarkers, interpretation of test results, and limitations associated with these testing methods, especially in the context of gynecologic cancers. The recommendations for immunotherapy testing of biomarkers based on tumor type and studies supporting these recommendations are also discussed and summarized. The Mini-Module ends with a discussion between Dr. Mills and Nora Disis, MD, FACP, of the University of Washington on the potential of total tumor DNA sequencing for detecting biomarkers
Run time: Approximately 20 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants, and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described
in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: September 29, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | 
 Gynecologic Cancer CPG Mini-Module – Single agent immunotherapy for Cervical Cancer and Combination Therapy for Mismatch Repair-Proficient (pMMR) Endometrial Cancer Gynecologic Cancer CPG Mini-Module – Single agent immunotherapy for Cervical Cancer and Combination Therapy for Mismatch Repair-Proficient (pMMR) Endometrial Cancer
The SITC Cancer Immunotherapy Guidelines Webinars connect clinicians with leading experts in the field and provide education about the evidence- and consensus-based recommendations in the clinical practice guidelines. To complement the advanced live webinar series, three on-demand, mini modules will assist learners in applying lessons learned on specific topics within the use of immunotherapy in their own practices and locate additional resources to continue their education.
Claire Friedman, MD, of Memorial Sloan Kettering Cancer Center presents two case studies comparing and contrasting single-agent immunotherapy and immunotherapy-based combination therapy for gynecologic cancers. The first study presented addresses pembrolizumab monotherapy for recurrent high-risk HPV-positive cervical cancer, and the second presentation discusses the use of the tyrosine kinase inhibitor lenvatinib plus pembrolizumab to treat recurrent pMMR endometrial cancer. Dr. Friedman presents the relevance of the current guidelines to these cases, key studies supporting the recommendations, clinical benefits and risks associated with single agent therapies versus combination therapies, and future challenges facing the immuno-oncology field for gynecologic cancers. Management of adverse events associated with targeted therapies and/or immunotherapies is also addressed. Dr. Friedman, Dr. Bajpai, and Dr. Odunsi conclude the Mini-Module by discussing practical approaches to promote patient tolerance of lenvatinib.
Run time: Approximately 15 minutes
Target Audience
Clinicians and advanced practice providers who treat cancer patients are the target audience, including community physicians, oncologists, emergency room physicians, disease specialists, registered nurses, nurse practitioners, pharmacists, physician assistants and radiologists.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
The Quick Education Modules are part of the Cancer Immunotherapy Clinical Practice Guidelines Advanced Webinar Series supported, in part, by grants from Amgen and Merck & Co., Inc. (as of 09/07/2023).
.png)
The SITC Clinical Practice Guidelines are produced and funded solely by SITC. No outside funding is received for the development of the manuscripts.

| | Original Course Date: September 29, 2023
On-Demand Release Date: Available Now | MORE INFO |
 | Published: On 08/11/2023
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
The course- Combination therapies in Combating Cancer gives a brief overview of the several combination therapies such as Inhibitory Immune Checkpoint Combination therapy, Targeted Immunotherapy approches, and other emerging combination therapies. In this course, the subject matter expert describes the effectiveness of various combination immunotherpy approches and their outcomes in cancer patients using several case studies and research papers. The course content emphasizes the emerging landscape of combining immunotherapy with different modalities to treat cancer.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete activity: 1 hour
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe key immunotherapy targets that are useful for combination approaches.
- Describe immunotherapy-based combination treatment regimens currently under investigation.
- Discuss the emerging landscape of combining immunotherapy with different modalities to treat cancer.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty
Gregory B. Lesinski, PhD, MPH
Co-Director, Translational GI Malignancy Program
Winship Cancer Institute of Emory University,
Atlanta, Georgia, USA
Disclosure of Conflicts of Interest
The faculty reported the following relevant financial relationships with ineligible entities related to the education content of this CE activity:
Gregory B. Lesinski, PhD, MPH: Works at a Academic Medical Center and a consultant advisor speaker for Bristol-Myers Squib, Vaccinex, Boerhinger-Ingelheim, Merck and Co.
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: August 11, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published Date: 06/30/2023
Expiration Date: 06/30/2024
Jointly provided by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC).
The 2023 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. The course, Introduction to Biomarkers provides an overview on types of biomarkers and its significance in treatment of cancer. The SME has discussed various findings and outcomes of the clinical trial initiatives related to biomarkers.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete the activity: 45 minutes
For additional information about the accreditation of this activity, please visit https://partnersed.com
Hardware and Software Requirements
SITC ConnectEd requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, and other healthcare providers who care for patients with cancer.
Educational Objectives:
Upon completion of this activity, participants should be able to:
- Define the term biomarker.
- Explain the functions of biomarkers.
- Differentiate the biomarkers based on their types.
- Describe the use of biomarkers in various cancer treatments.
- Summarize the challenges of using biomarkers in the treatment of cancer.
Faculty and Disclosure of Conflicts of Interest
PACE requires planners, faculty, and others who are in a position to control the content of this activity to disclose all financial relationships they may have with ineligible companies. All relevant financial relationships are thoroughly vetted and mitigated according to PACE policy. PACE is committed to providing learners with high-quality accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Faculty:
Dr. Paolo A. Ascierto, MD
Director of Department of Melanoma
UOC Melanoma, Immunoterapia Oncologica e Terapie Innovative
Istituto Nazionale Tumori – Fondazione “G. Pascale”
Napoli, Italy
The faculty reported the following financial relationships or relationships to products or devices they have with ineligible companies:
Conflicts of Interest:
Researcher: Bristol Myers Squibb, Roche-Genentech, Pfizer, Sanofi
Consultant/Advisor/Speaker: Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre-Fabre, AstraZeneca, Sun Pharma, Sanofi, Idera, Sandoz, Immunocore, 4SC, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, iTeos, Medicenna, Bio-Al Health, ValoTX, Replimmune, Bayer
The PACE planners and others have no relevant financial relationship(s) to disclose with ineligible companies. The SITC planners and others have no relevant financial relationship(s) to disclose with ineligible companies.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
PACE designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 0.75 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-23-162-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Instructions for Credit
During the period 06/30/2023 through 06/30/2024, participants must read the learning objectives and faculty disclosures and study the educational activity. Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
| | Original Course Date: June 30, 2023
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 0.75 hours Contact HourANCC: 0.75 hours Contact HourCoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published Date: 06/01/2023
Expiration Date: 06/01/2024
Jointly provided by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC).
The 2023 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. The course, Mechanisms of Immune-related Adverse Events (irAEs) provides an insight into the host factors that increase the risk of irAEs. The course briefly explains the differences in toxicities of CTLA-4 and PD-1 immune checkpoint inhibitors. The two case studies presented in course enables the learners to assess the immune-related response of immunotherapy treatment in a patient.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete the activity: 45 minutes
For additional information about the accreditation of this activity, please visit https://partnersed.com
Hardware and Software Requirements
SITC ConnectEd requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, and other healthcare providers who care for patients with cancer.
Educational Objectives:
Upon completion of this activity, participants should be able to:
- Explain the Mechanisms of immune related Adverse Events in brief.
- Describe the other host factors that affect the risk of immune related Adverse Events.
- Summarize the differences in PD-L1 and CTLA-4 toxicities.
- Explain the response assessment process in a patient for the immunotherapy treatment.
Faculty and Disclosure of Conflicts of Interest
PACE requires planners, faculty, and others who are in a position to control the content of this activity to disclose all financial relationships they may have with ineligible companies. All relevant financial relationships are thoroughly vetted and mitigated according to PACE policy. PACE is committed to providing learners with high-quality accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Faculty:
Pradnya Patil, MD, FACP
Associate Staff Oncologist
Taussig Cancer Center, Cleveland Clinic
Cleveland, OH
The faculty reported the following financial relationships or relationships to products or devices they have with ineligible companies:
Conflicts of Interest:
Consultant/Advisor/Speaker: AstraZeneca, Jazz Pharmaceuticals
The PACE planners and others have no relevant financial relationship(s) to disclose with ineligible companies. The SITC planners and others have no relevant financial relationship(s) to disclose with ineligible companies.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
PACE designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 0.75 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-23-151-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Instructions for Credit
During the period 06/01/2023 through 06/01/2024 participants must read the learning objectives and faculty disclosures and study the educational activity. Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
| | Original Course Date: May 30, 2023
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 0.75 hours Contact HourANCC: 0.75 hours Contact HourCoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published Date: 05/05/2023
Expiration Date: 05/05/2024
Jointly provided by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC).
The 2023 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team. The ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
This course, Introduction to Immunology provides an overview of the immune system, focusing on the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The foundational knowledge presented in this course is critical to understanding how the immune system can be harnessed to treat cancer through immunotherapy.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete the activity: 1 hour
For additional information about the accreditation of this activity, please visit https://partnersed.com
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, and other healthcare providers who care for patients with cancer.
Educational Objectives:
Upon completion of this activity, participants should be able to:
- Describe the function of the components of the immune system, including relevant cells, molecules, and organs of the immune system.
- Differentiate between the adaptive immune system and the innate immune system.
- Recognize the barriers to effective immunotherapy, including mechanism by which tumors locally disable and/or evade the immune system.
Faculty and Disclosure of Conflicts of Interest
PACE requires planners, faculty, and others who are in a position to control the content of this activity to disclose all financial relationships they may have with ineligible companies. All relevant financial relationships are thoroughly vetted and mitigated according to PACE policy. PACE is committed to providing learners with high-quality accredited CE activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of an ineligible company.
Faculty:
Chrystal M. Paulos, PhD
Director of Translational Research, Cutaneous Malignancies
Winship Cancer Institute at Emory University
The faculty reported the following financial relationships or relationships to products or devices they have with ineligible companies:
Conflicts of Interest:
IP Rights: Ares Immunotherapy
Consulting Fees: Ares Immunotherapy
Founder/Owner: Ares Immunotherapy
Other (subcontracts): Obsidian, Lycera, ThermaFisher
The PACE planners and others have no relevant financial relationship(s) to disclose with ineligible companies. The SITC planners and others have no relevant financial relationship(s) to disclose with ineligible companies.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (PACE) and Society for Immunotherapy of Cancer (SITC). PACE is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
PACE designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.0 contact hours. Designated for 0.5 contact hours of pharmacotherapy credit for Advance Practice Registered Nurses.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.0 contact hour(s) (0.1 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-23-142-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Instructions for Credit
During the period 05/05/2023 through 05/05/2024 participants must read the learning objectives and faculty disclosures and study the educational activity. Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
| | Original Course Date: May 05, 2023
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1 hour Contact HourANCC: 1 hour Contact HourCoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Published: On 04/28/2023
Please note, this activity does not offer continuing education credit.
The 2023 Advances in Cancer Immunotherapy™ educational series is supported, in part, through independent medical education grants from AstraZeneca, Bristol Myers Squibb, Exelixis, GSK, Merck Sharp & Dohme, Corp., a subsidiary of Merck & Co., Inc. (MSD) and Novartis Pharmaceuticals Corporation.
Additional support for this activity provided by:

Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future.
This course, Basic Principles of Cancer Immunotherapy will walk the learner through the biological foundations of immunotherapy, types of immunotherapies, illustrate how biomarkers play a major role in immunotherapy, as well as cover the patient response to immunotherapy and subsequent physician assessment.
View all available ACI online offerings at www.sitcancer.org/acionline.
Estimated time to complete activity: 1 hour
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the concept of Immunotherapy.
- Identify the various types of cancer immunotherapy.
- Describe the rationale for common approaches to cancer immunotherapy.
- Explain the response assessment process in a patient for the immunotherapy treatment.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form/Survey to receive a certificate of completion.
Faculty
Christian M. Capitini, MD
Associate Professor
University of Wisconsin-Madison
Disclosure of Conflicts of Interest
The faculty reported the following relevant financial relationships with ineligible entities related to the education content of this CE activity:
Christian M. Capitini, MD: Works at a Academic Medical Center and a consultant advisor speaker for Bayer, Elephas, Novartis, Nektar Therapeutics, WiCell Research Institute
The Society for Immunotherapy of Cancer planners and others have nothing to disclose.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all the information and data before treating patients or employing any therapies described in this educational activity. Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
| | Original Course Date: April 28, 2023
|
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Please note: this course is no longer eligible for continuing education credits.
Published December 24, 2020
Course Description
This interactive course provides an overview of the mechanisms by which cancer immunotherapy may activate immune-related adverse events (irAEs). The foundational knowledge presented in this course is critical to understanding how and why immune-related adverse events occur and how they can be treated.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Pradnya Patil, MD, FACP
Hematology/Oncology Fellow
Taussig Cancer Institute, Cleveland Clinic
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Discuss the mechanisms of peripheral and central immune tolerance.
- Describe the biological pathways involved in mediating autoimmune reactions.
- Explain the differences between PD-1 and CTLA-4 pathway-mediated autoimmunity.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: December 24, 2020 - December 24, 2021
Estimated time to complete activity: 0.5 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2020-2021 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol Myers Squibb, Exelixis, Inc., and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2520-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Pradnya Patil, MD, FACP: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: December 24, 2020
|
Approved Credit: ACPE: 0.50 hours Contact HourANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 2: Basic Cancer Immunotherapy Concepts, will cover the biological foundation of cancer immunotherapy, applying the basic immunology you learned in the last module to help lay the foundation for future topics in this program.
- Original Release Date: October 26, 2020
- Expiration Date: October 27, 2026
- Date of Last Review: October 2023
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Click on image for FREE PREVIEW
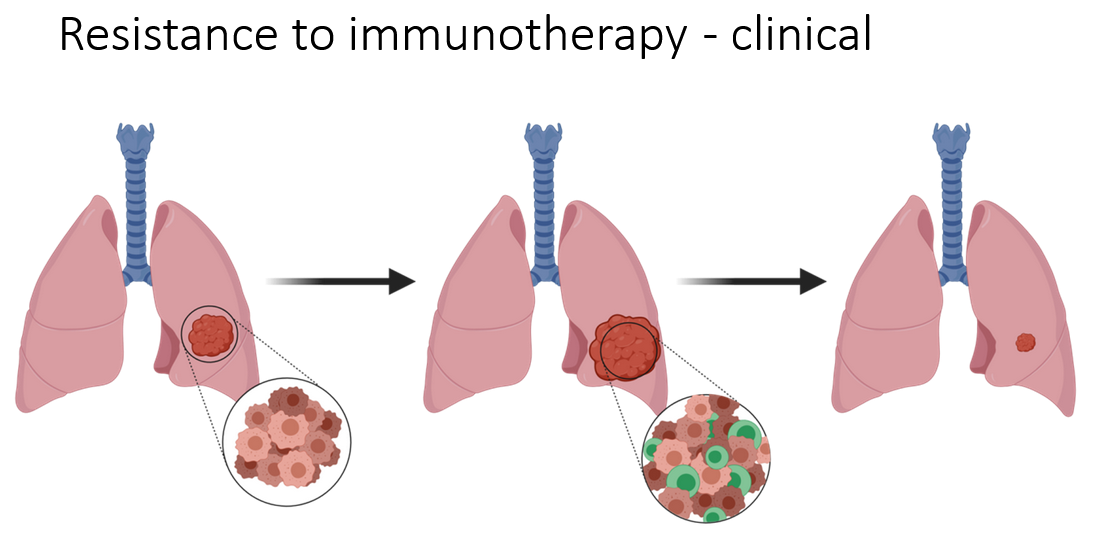
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
At the conclusion of this activity, participant should be able to: |
| Basis of Tumor Immunosurveillance and Immunotherapy |
- Describe how the immune system recognizes and eliminates cancer cells, including the concept of immunologic memory.
- Characterize the function of specific immune cell populations in mediating tumor immunotherapy and/or immune suppression.
- Distinguish between immunosurveillance, immunoediting and immunotherapy of cancer.
- Identify the implications of immune depleted, excluded and infiltrated tumor microenvironments.
- Identify how both central and peripheral tolerance can impact immunosurveillance and immunotherapy.
- Describe the difference between primary and acquired resistance to immunotherapy.
- Apply concepts of the cancer-immunity cycle to identify how biomarkers inform and influence tumor immunotherapy.
|
| Immunologic Effects of Other Therapies |
- Describe the immunologic effects of standard cancer therapeutics on the tumor microenvironment and immune system.
- Describe the potential impact of other, non-cancer agents, on tumor immunotherapy responses and toxicities.
- Characterize the impact of standard cancer treatments on the efficacy of cancer immunotherapy.
- Characterize the impact of standard cancer treatments on the toxicity of cancer immunotherapy.
- Distinguish total body versus focal radiotherapy effects.
- Recognize the impact of intratumoral injection and other local therapies on the tumor microenvironment and anti-tumor immune response.
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Hussein Tawbi, MD, PhD
Deputy Chair and Professor, Department of Melanoma Medical Oncology
Director of Melanoma Clinical Research & Early Drug Development
Co-Director, MD Anderson Brain Metastasis Clinic
University of Texas MD Anderson Cancer Center, Houston, TX
|
- Consultant: Genentech, BMS, Novartis, Merck, Array
- Contracted Research: Genentech, BMS, Novartis, Merck, GSK
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-23-272-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 10/27/2023 through 10/27/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: October 26, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1.50 hours Contact HourANCC: 1.50 hours Contact Hour
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version go here to find the Immunotherapy for the Treatment of Breast and Gynecologic Cancers that is a CME-, CPE-, CNE- and MOC-certified activity.
Published August 11, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Breast and Gynecologic Cancers, provides an overview of the still emerging applications of Breast and Gynecologic Cancer Immunotherapy. Several clinical trials are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for Breast and Gynecologic Cancer.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Evanthia Roussos Torres, MD, PhD
Assistant Professor of Medicine, Oncology
University of Southern California
Norris Comprehensive Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the early stage application of breast and gynecologic cancer immunotherapy treatment in relation to standard-of-care therapies
- Implement cancer immunotherapy treatment for breast and gynecologic cancer and better manage common side effects
- Describe the rationale for common approaches to cancer immunotherapy
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 8/11/20 - 8/11/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2249-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.5 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Evanthia Roussos Torres, MD, PhD: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 8/11/20 through 8/11/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Breast & Gynecologic Cancers.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: August 11, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Please note, this course is no longer eligible to receive CE credits.
Published July 29,2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Microsatellite Instability (MSI) – High Cancers, provides background on MSI and an overview of immunotherapy treatment for MSI-high populations. Several clinical trials are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for MSI-high cancers.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
John C. Leighton, Jr., MD
Division Chair, Hematology and Medical Oncology
Einstein Healthcare Network
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe microsatellite instability and the implications for immunotherapy treatment
- Provide cancer immunotherapy treatment for microsatellite instability – high cancers
- Describe the rationale for common approaches to cancer immunotherapy
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 7/29/20 - 7/29/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2214-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.2 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
John C. Leighton, Jr., MD: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 7/29/20 through 7/29/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Microsatellite Instability (MSI) – High Cancers.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: July 29, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 |  Immunotherapy has permeated the cancer treatment landscape among several tumor types and treatment settings. With key late-stage clinical trials underway, immunotherapy experts provide critical updates on emerging data, recent approvals, and promising immunotherapy combinations with other classes of agents. Updates will be provided across solid tumors with opportunities to ask critical clinical questions and receive expert answers. Immunotherapy has permeated the cancer treatment landscape among several tumor types and treatment settings. With key late-stage clinical trials underway, immunotherapy experts provide critical updates on emerging data, recent approvals, and promising immunotherapy combinations with other classes of agents. Updates will be provided across solid tumors with opportunities to ask critical clinical questions and receive expert answers.
This activity is intended for medical, surgical, radiation oncologists and other healthcare professionals (i.e. oncology nurses, physicians’ assistants, pharmacists, and others) interested/involved in the care of patients with cancer, who are being treated with immunotherapy.

Learning Objectives:
- Examine the relevance of phase II and III trial data regarding emerging indications in solid tumors for immune checkpoint inhibitors
- Assess latest clinical trial data on immune checkpoint inhibitors across a wide range of tumors and implement new practice standards accordingly
- Recognize the rationale(s) behind combining checkpoint inhibitors with other anti-cancer drugs, including chemotherapy, targeted therapies, and other immunotherapy
- Plan to effectively implement evidence-based new approaches of combination regimens with checkpoint inhibitors as they become available
Approximate Time to Complete: 60 minutes
Credit Available: June 10, 2020 - February 28, 2021
Developed through a collaboration between SITC and PlatformQ.
| | Original Course Date: July 24, 2020
|
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 |  Effective survivorship care requires coordination among a team of specialists, as well as counseling and education that enables patients to effectively manage their post-treatment health and well-being. As the long-term effects of checkpoint inhibitors are incorporated into survivorship planning, a multidisciplinary panel will discuss evidence-based strategies to provide a framework to address the unique needs of patients treated with immunotherapy. Effective survivorship care requires coordination among a team of specialists, as well as counseling and education that enables patients to effectively manage their post-treatment health and well-being. As the long-term effects of checkpoint inhibitors are incorporated into survivorship planning, a multidisciplinary panel will discuss evidence-based strategies to provide a framework to address the unique needs of patients treated with immunotherapy.
This activity is intended for medical, surgical, radiation oncologists; primary care physicians; internal medicine specialists; health care professionals including oncology pharmacists, oncology advanced practice nurses and physician assistants, and other health care providers who participate in the care of patients with cancer.

Learning Objectives:
- Apply recent guidelines and expert recommendations for the creation of comprehensive survivorship plans for patients who have received immune checkpoint inhibitors
- Plan strategies on developing survivorship plans within your multidisciplinary cancer care team
Approximate Time to Complete: 60 minutes
Credit Available: June 10, 2020 - February 28, 2021
Developed through a collaboration between SITC and PlatformQ.
| | Original Course Date: July 24, 2020
|
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 |  While it is well established that immunotherapies have unique toxicity profiles, effective management is not clearly defined and a challenge for many cancer teams. As evidence emerges on severity and onset of immune-related adverse events (irAEs), it is imperative that clinicians stay abreast of strategies to improve awareness, risk-assessment, monitoring, and management of irAEs including dosing modifications for immunotherapies. Experts will discuss these critical issues as well as the importance of a coordinated approach with multiple specialists for effective irAE management. While it is well established that immunotherapies have unique toxicity profiles, effective management is not clearly defined and a challenge for many cancer teams. As evidence emerges on severity and onset of immune-related adverse events (irAEs), it is imperative that clinicians stay abreast of strategies to improve awareness, risk-assessment, monitoring, and management of irAEs including dosing modifications for immunotherapies. Experts will discuss these critical issues as well as the importance of a coordinated approach with multiple specialists for effective irAE management.
This activity is intended for medical, surgical, radiation oncologists; primary care physicians; internal medicine specialists; health care professionals including oncology pharmacists, oncology advanced practice nurses and physician assistants, and other health care providers who participate in the care of patients with cancer.

Learning Objectives:
- Anticipate unique and common immune-related adverse events associated with immunotherapy across a wide range of solid tumors
- Implement expert-recommended best practices for managing adverse effects that can occur with immunotherapy
Approximate Time to Complete: 60 minutes
Credit Available: June 10, 2020 - February 28, 2021
Developed through a collaboration between SITC and PlatformQ.
| | Original Course Date: July 24, 2020
|
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version, go here to find the Immunotherapy for the Treatment of Lung Cancer that is a CME-, CPE-, CNE-, and MOC-certified activity.
Published July 15, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Lung Cancer, provides an overview of immunotherapy treatment for lung cancer. Multiple clinical trials dealing with both non-small cell lung cancer and small cell lung cancer are discussed, as are key findings that have resulted in recent FDA approvals. Learners will have an opportunity to interactively apply what they've learned through an in-depth case study. At the end of this course, participants will be better able to implement immunotherapy treatment for lung cancers.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Deepa Rangachari, M.D.
Assistant Professor of Medicine, Harvard Medical School
Thoracic Oncology Program, Beth Israel Deaconess Medical Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Implement cancer immunotherapy treatment for lung cancer.
- Identify the appropriate clinical management of common side effects of immunotherapy agents.
- Describe the rationale for common approaches to cancer immunotherapy.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: July 15, 2020 - July 15, 2021
Approximate Time to Complete: 60 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.0 contact hour (0.1 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2190-H01)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.7 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Deepa Rangachari, MD: Contracted research support from Bristol Myers Squibb, Novocure, and Abbvie/Stemcentrx; consulting fees from Astra Zeneca, DynaMed, and Advance Medical/Teladoc Health
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 7/15/20 through 7/15/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Lung Cancer.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: July 15, 2020
|
Approved Credit: ACPE: 1 hour Contact HourANCC: 1 hour Contact HourACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Access Course
 As diagnostic and therapeutic advances intersect, there is a significant impact on oncology patient care and increased the use of molecular testing to guide treatment decision-making. While these practices are evidence-based, complex, and incorporated into clinical guidelines, their adoption is fraught with challenges including the multiple companion diagnostic tests associated with immunotherapies. Ask an expert pathologist and oncologist your burning questions as they discuss their strategies for improving molecular testing, which biomarkers to test for, interpreting molecular test results, and how that translates to selecting immunotherapy for patients with solid tumors. As diagnostic and therapeutic advances intersect, there is a significant impact on oncology patient care and increased the use of molecular testing to guide treatment decision-making. While these practices are evidence-based, complex, and incorporated into clinical guidelines, their adoption is fraught with challenges including the multiple companion diagnostic tests associated with immunotherapies. Ask an expert pathologist and oncologist your burning questions as they discuss their strategies for improving molecular testing, which biomarkers to test for, interpreting molecular test results, and how that translates to selecting immunotherapy for patients with solid tumors.
This activity is intended for medical, surgical, radiation oncologists and other healthcare professionals (i.e. oncology nurses, physicians’ assistants, pharmacists, and others) interested/involved in the care of patients with cancer, who are being treated with immunotherapy.
Learning Objectives:
- Examine the variety of biomarkers associated with immune checkpoint inhibitor therapy
- Select appropriate, evidence-based testing modalities for patients who are potential candidates for immune checkpoint inhibitor therapy and interpret testing results effectively
Approximate Time to Complete: 60 minutes
Credit Available: June 10, 2020 - February 28, 2021
Developed through a collaboration between SITC and PlatformQ.
| | Original Course Date: June 10, 2020
|
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | This course is no longer available for credit.
Published May 26, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Skin Cancers, provides the latest in immunotherapy treatment for skin cancers, and summarizes many of the key findings that have resulted in recent FDA approvals for this kind of treatment. Several clinical trials are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for skin cancers.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Ryan J. Sullivan, MD
Assistant Professor of Medicine
Harvard Medical School, Boston, MA
Division of Hematology/Oncology, Department of Medicine
Massachusetts General Hospital, Boston MA
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatment for skin cancers.
- Identify common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 5/26/20 - 5/26/21
Approximate Time to Complete: 75 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.25 AMA PRA Category 1 Credits™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.25 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.25 contact hours (.125 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2143-H01-P)
Type of Activity: Application
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.3 contact hours. Designated for 1.0 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Ryan J. Sullivan, MD: Consulting Fees (Merck, Novartis, Replimune, Bristol Myers Squibb, Asana Biosciences); Contracted Research (Merck, Amgen)
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 5/26/20 through 5/26/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Skin Cancers.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: May 26, 2020
|
Approved Credit: ACPE: 1.25 hours Contact HourANCC: 1.30 hours Contact HourACCME (MD/DO): 1.25 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.25 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1.25 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This course is no longer available for credit.
Originally published May 26, 2020 (Updated June 9. 2020)
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Hepatocellular Carcinoma (HCC), provides an overview of liver immunobiology and immunotherapeutic strategies in HCC, including checkpoint inhibition, blocking inhibitory cytokines, vaccine therapies, oncolytic viruses, and adoptive cell therapy. Several clinical trials are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for HCC.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Anuradha Krishnamurthy, MD
Assistant Professor of Medicine
University of Pittsburgh School of Medicine
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the characteristics of liver immunobiology
- Implement cancer immunotherapy treatment for HCC and better manage common side effects
- Describe the rationale for common approaches to cancer immunotherapy
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 5/26/20 - 5/26/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2142-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.5 contact hour of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Anuradha Krishnamurthy, MD: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 5/26/20 through 5/26/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Hepatocellular Carcinoma.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: May 26, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Access Course
 The role of immunotherapy in the treatment of patients with advanced squamous cell carcinoma of the head and neck (SCCHN) is explored through two cases. The role of immunotherapy in the treatment of patients with advanced squamous cell carcinoma of the head and neck (SCCHN) is explored through two cases.
The cases presented are modeled on the interactive grand rounds approach. The questions within the activity are designed to test your current knowledge. After each question, you will be able to see whether you answered correctly and read evidence-based information that supports the most appropriate answer choice.
This activity is intended for hematologist/oncologists, surgeons, and other healthcare professionals who treat patients with SCCHN.
The goal of this activity is to increase understanding of current approaches to treating advanced SCCHN with immunotherapy.
Learning Objectives:
- Have increased knowledge regarding the clinical study designs, as well as efficacy and safety findings, from trials that have evaluated immune checkpoint inhibitor regimens in patients with recurrent or metastatic SCCHN.
- Have greater competence related to identifying tumor characteristics and other clinical features that guide treatment selection in patients with recurrent or metastatic SCCHN.
- Have greater competence related to strategies for monitoring and managing treatment-related adverse events.
Approximate Time to Complete: 60 minutes
Credit Available: May 15, 2020 - May 15, 2021
Developed through a partnership between SITC and Medscape.
Additional Resources for Clinicans from SITC:

"The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC)" (Published July 15, 2019)
View Guidelines Here.
View On-Demand Webinar Here.
| | Original Course Date: May 15, 2020
|
Approved Credit: : 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | Access Course
 Experts discuss the current role of checkpoint inhibition in hepatocellular cancer (HCC) as well as the emergence of combination therapies. Experts discuss the current role of checkpoint inhibition in hepatocellular cancer (HCC) as well as the emergence of combination therapies.
The goal of this activity is to increase understanding of the latest advances in the use of immunotherapy for the treatment of HCC.
This activity is intended for hematologists/oncologists, gastroenterologists, nurses/nurse practitioners, pharmacists, and other healthcare professionals who treat patients with advanced hepatocellular cancer.
Learning Objectives:
- Have increased knowledge regarding the clinical trial data on the use of immune checkpoint inhibitors (ICIs) in managing unresectable HCC.
- Have greater competence related to identifying when an ICI should be considered for a patient with unresectable/advanced HCC.
- Have greater competence related to managing immune-related adverse events (irAEs) in patients with unresectable HCC.
Approximate Time to Complete: 45 minutes
Credit Available: May 8, 2020 - May 8, 2021
Developed through a partnership between SITC and Medscape.
Additional Resources for Clinicans from SITC:

View Guidelines Here.
| | Original Course Date: May 08, 2020
|
Approved Credit: : 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ANCC: 0.75 hours Contact HourACPE: 0.75 hours CEU
| MORE INFO |
 | Originally published April 30, 2020 (updated May 7, 2020)
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Head and Neck Cancer, provides an overview of immunotherapy treatment for head and neck cancer. Multiple clinical trials are discussed, including both mono- and combination therapies. Learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for head and neck cancer.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Michael Gibson, MD, PhD FACP
Associate Professor of Medicine
Director of Translational Research for Head and Neck Oncology
Director of Translational Therapeutics for Esophago-Gastric Oncology
Vanderbilt-Ingram Cancer Center
Vanderbilt University Medical Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatments for head and neck cancers.
- Identify appropriate clinical management of common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 4/30/20 - 4/30/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2066-H01-P)
Type of Activity: Application
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hour. Designated for 0.5 contact hour of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Michael Gibson, MD, PhD: Consulting fees to Merck, Bristol-Myers Squibb; Fees for Non-CME/CE services received directly from a commercial interest or their agents to Bristol-Myers Squibb (non-branded)
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 4/30/20 through 4/30/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Head and Neck Cancer.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Firefox, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: April 30, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Access Course
 Enhance your clinical skills as you engage with patients in an objective and realistic interactive simulation. Treat patients, diagnose medical conditions, and prescribe medications. There are no limits to your choices - order from thousands of labs, diagnoses, devices, and drugs using an intuitive patient chart-based interface. Enhance your clinical skills as you engage with patients in an objective and realistic interactive simulation. Treat patients, diagnose medical conditions, and prescribe medications. There are no limits to your choices - order from thousands of labs, diagnoses, devices, and drugs using an intuitive patient chart-based interface.
Powerful metrics track your performance and provide instant expert feedback on your decisions. Upon completion, results will be presented, along with author commentary on each case.
This activity is intended for Hem/Onc specialists, urologists, and emergency medicine specialists.
The goal of this activity is to provide clinicians with the latest data, concepts, and recommendations regarding the diagnosis, evidence-based treatment, counseling, and follow-up related to patients with advanced urothelial cancer.
Learning Objectives:
- Have greater competence related to ordering appropriate testing to determine patient eligibility for therapy in advanced urothelial carcinoma (UC).
- Have greater competence related to prescribing appropriate therapy in a patient with advanced UC based on patient and disease-specific factors.
- Have greater competence related to providing appropriate conseling and follow-up for a patient receiving treatment for advanced UC.
Approximate Time to Complete: 60 minutes
Credit Available: April 28, 2020 - April 28, 2021
Developed through a partnership between SITC and Medscape.
Additional Resources for Clinicans from SITC:

"The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma" (Published August 15, 2017)
View Guidelines Here.
| | Original Course Date: April 28, 2020
|
Approved Credit: : 1.25 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACCME (MD/DO): 1.25 hours AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | Access Course
 Read about the latest advances in immuno-and targeted therapies for treating patients with advanced cervical and endometrial cancers. Read about the latest advances in immuno-and targeted therapies for treating patients with advanced cervical and endometrial cancers.
This activity is intended for hematologist/oncologists, obstetrics/gynecologists, and other healthcare professionals who treat patients with cervical and endometrial cancers.
The goal of this activity is to increase understanding of current standards and new strategies for the treatment of advanced cervical and endometrial cancers.
Learning Objectives:
- Have increased knowledge regarding the current standard of care for patients with advanced cervical and endometrial cancers.
- Have increased knowledge regarding the clinical trial safety and efficacy data for novel therapies, both alone and in combination with other novel agents, in advanced cervical and endometrial cancers.
- Demonstrate greater confidence in their ability to interpret clinical trial data of novel agents in order to understand the impact on the treatment paradigm in advanced cervical and endometrial cancers.
Approximate Time to Complete: 60 minutes
Credit Available: April 10, 2020 - April 10, 2021
Developed through a partnership between SITC and Medscape.
Additional Resources for Clinicans from SITC:

View Available Guidelines Here.
| | Original Course Date: April 10, 2020
|
Approved Credit: : 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | Access Course
 Experts discuss the use of checkpoint inhibitors in the first and second line for advanced urothelial carcinoma as well as future directions. Experts discuss the use of checkpoint inhibitors in the first and second line for advanced urothelial carcinoma as well as future directions.
This activity is intended for oncologists, urologists, emergency department physicians, and other healthcare professionals who treat patients with bladder cancer.
The goal of this activity is to increase understanding in the latest advances in immunotherapy for the treatment of patients with advanced bladder cancer.
Learning Objectives:
- Have increased knowledge regarding the current role for immune checkpoint inhibitors (ICIs) in the treatment of advanced urothelial carcinoma (UC).
- Have increased knowledge regarding novel ICIs and novel combination trials in advanced UC.
- Have greater competence related to incorporating emerging data into clinical practice when managing patients with advanced UC.
Approximate Time to Complete: 60 minutes
Credit Available: April 8, 2020 - April 8, 2021
Developed through a partnership between SITC and Medscape.
Additional Resources for Clinicans from SITC:

"The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of bladder carcinoma" (Published August 15, 2017)
View Guidelines Here.
| | Original Course Date: April 08, 2020
|
Approved Credit: : 1.25 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACCME (MD/DO): 1.25 hours AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | This course is no longer available for credit. Please refer to the newest version of Immunotherapy for the Treatement of Genitourinary Malignancies, published on 11/1/2021.
Originally published March 31, 2020 (updated May 7, 2020)
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Genitourinary Malignancies, provides an overview of immunotherapy treatment for the genitourinary malignancies renal cell carcinoma, urothelial carcinoma, and prostate cancer. Multiple clinical trials are discussed, including both mono- and combination therapies. Learners will have an opportunity to interactively apply what they've learned through three case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for genitourinary malignancies.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Pedro Barata, MD, MSc
Assistant Professor of Medicine
Deming Department of Medicine
Section of Hematology/Oncology
Tulane University School of Medicine
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatments for genitourinary malignancies.
- Identify appropriate clinical management of common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 3/31/20 - 3/31/21
Approximate Time to Complete: 1 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.0 contact hour (0.1 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-2051-H01-P)
Type of Activity: Application
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.0 contact hour. Designated for 0.8 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Pedro Barata, MD, MSc: Consulting Fees (Exelixis, Caris, Bayer, Janssen, EMD-Serono, Pfizer, Astellas, Dendreon, Clovis, Eisai, Sanofi); Contracted Research (BlueEarth Diagnostics, Nektar, AstraZeneca and Seattle Genetics)
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 3/31/20 through 3/31/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Genitourinary Malignancies.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Firefox, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: March 31, 2020
|
Approved Credit: ACPE: 1 hour Contact HourANCC: 1 hour Contact HourACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version, go here to find the What's Next in Cancer Immunotherapy that is a CME-, CPE-, CNE-, and MOC-certified activity.
Published February 11, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, What's Next for Cancer Immunotherapy?, covers the future of cancer immunotherapy such as understanding important trends, new developments, potential approvals and disease states with promising data.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Evan J. Lipson, MD
Associate Professor, Department of Oncology
Johns Hopkins University School of Medicine
Parul Agarwal, MD
Medical Oncology Fellow
Johns Hopkins University School of Medicine
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale behind expanding the settings in which cancer immunotherapy is administered, including patients with a larger variety of tumor types and patient populations previously excluded from clinical trial participation.
- Describe the basis for novel immunotherapy drug combinations.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 2/11/20 - 2/11/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-1083-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.4 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Evan J. Lipson, MD: Consultant (Bristol-Myers Squibb, Novartis, EMD Serono, Array BioPharma, Macrogenics, Merck); Contracted Research (Bristol-Myers Squibb, Merck)
Parul Agarwal, MD: Nothing to Disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 2/11/20 through 2/11/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for What's Next for Cancer Immunotherapy?
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: February 11, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Please note this course is no longer available for credit. Click here to access the current version of Immunotherapy for the Treatment of Hematologic Malignancies which is a CME-, CPE-, CNE-, and MOC-certified activity.
Originally published January 23, 2020 (Updated May 7, 2020)
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Immunotherapy for the Treatment of Hematologic Malignancies, provides the latest in immunotherapy treatment for hematological cancers, and summarizes many of the key findings that have resulted in recent FDA approvals for this kind of treatment. Several clinical trials are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to implement immunotherapy treatment for hematologic cancers.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Bhagirathbhai R. Dholaria, MBBS
Assistant Professor
Department of Hematology - Oncology
Vanderbilt University School of Medicine, Nashville, TN
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatment for hematologic cancers.
- Identify common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 1/23/20 - 1/23/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-1072-H01-P)
Type of Activity: Application
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.8 contact hours. Designated for 0.7 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Bhagirathbhai R. Dholaria, MBBS: Celgene (consulting)
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 1/23/20 through 1/23/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Immunotherapy for the Treatment of Hematologic Malignancies.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: January 23, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.80 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Published January 16, 2020
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Toxicity Management, provides an overview of clinical toxicity management for cancer immunotherapy. Both common and uncommon immune-related adverse events (irAEs) are discussed and learners will have an opportunity to interactively apply what they've learned through two case studies. At the end of this course, participants will be better able to manage immune-related adverse events for patients receiving cancer immunotherapy.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Morgan Gwynn, PharmD, BCOP, CPP
Melanoma and Head & Neck Oncology Clinical Pharmacist Practitioner
University of North Carolina Medical Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Identify common and uncommon immune-related adverse events associated with cancer immunotherapy
- Describe best practices for managing common side effects seen in cancer immunotherapy treatment
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 1/16/20-1/16/21
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-20-1068-H01-P)
Type of Activity: Application
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.8 contact hour. Designated for 0.7 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Morgan Gwynn, PharmD, BCOP, CPP: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 1/16/20 through 1/16/21 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Toxicity Management.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: January 16, 2020
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.80 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This version of the course is no longer available for credit. To access the accredited version go here to find the Mechanisms of Immune-Related Adverse Events that is a CME-, CPE-, CNE- and MOC-certified activity.
Published December 23, 2019
Course Description
This interactive course provides an overview of the mechanisms by which cancer immunotherapy may activate immune-related adverse events (irAEs). The foundational knowledge presented in this course is critical to understanding how and why immune-related adverse events occur and how they can be treated.
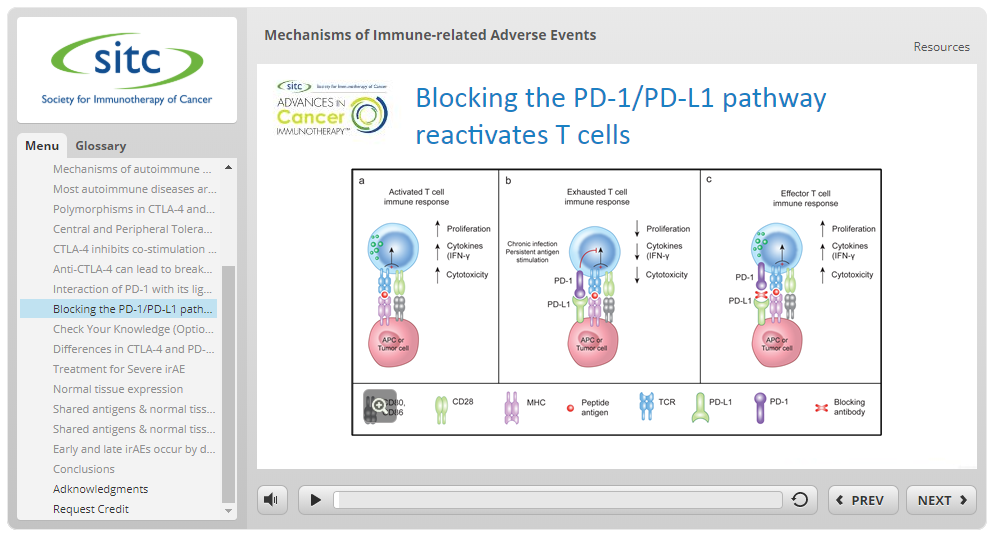
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Pradnya Patil, MD, FACP
Hematology/Oncology Fellow
Taussig Cancer Institute, Cleveland Clinic
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Discuss the mechanisms of peripheral and central immune tolerance.
- Describe the biological pathways involved in mediating autoimmune reactions.
- Explain the differences between PD-1 and CTLA-4 pathway-mediated autoimmunity.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: December 23, 2019 - December 23, 2020
Estimated time to complete activity: 0.5 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-887-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Pradnya Patil, MD, FACP: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period December 23, 2019 through December 23, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for "Mechanisms of Immune-Related Adverse Events."
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete the post-test with a passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: December 23, 2019
|
Approved Credit: ACPE: 0.50 hours Contact HourANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Published December 17, 2019
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This Basic Principles of Cancer Immunotherapy course covers the basic principles and mechanisms behind cancer immunotherapy.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses, and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Stefani Spranger, PhD
Assistant Professor of Biology
Koch Institute for Integrative Cancer Research at MIT
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy
-
Recognize various approaches to cancer immunotherapy including therapeutic vaccine, adoptive cell transfer, cytokines, checkpoint inhibitor modulation, effector antibodies and antibody-drug conjugates
-
Explain the mechanism by which tumors evade the immune system and mechanisms to overcome this immunosuppression
-
List important biomarkers for measuring the immune response
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 12/17/19-12/17/20
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-991-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.3 contact hours of pharmacotherapy credit for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Stefani Spranger, PhD: Consulting fees to Venn Therapeutic, Tango, Takeda, Replimune, Ribon, Dragonfly, Merck, Torque; Contracted Research to Takeda, Exelixis
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 12/17/19 through 12/17/20 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Basic Principles of Cancer Immunotherapy.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Firefox, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: December 17, 2019
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This course is no longer available for credit. Please access the updated course, Practical Barriers in Cancer Immunotherapy Treatment, which is a CME-, CPE-, CNE- and MOC-certified activity.
Published December 10, 2019
Course Description
This interactive online course is part of the Society for Immunotherapy of Cancer’s (SITC) Advances in Cancer Immunotherapy™ (ACI) program. Designed for oncologists, nurses, pharmacists, emergency physicians and all members of the cancer team, the ACI program focuses on recent clinical advancements, management of immune-related adverse events, underlying mechanisms of different immunotherapy options, clinical application for various disease states, and what to look for in the future. View all available ACI online offerings at www.sitcancer.org/acionline.
This course, Practical Barriers in Cancer Immunotherapy Treatment, provides a detailed overview of how to overcome barriers to obtaining reimbursement from both Medicare and commercial payers for the use of immunotherapy in cancer treatment.
At the end of this course, participants will be able to identify solutions to overcome both operational and financial barriers to integrating immunotherapy into their practice setting.
Target Audience
This activity is intended for program coordinators, health care managers, physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Amy Schippers, PA-C
Physician Assistant
St. Luke's University & Health Network
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Identify and implement solutions to overcome operational barriers to implementing cancer immunotherapy in the clinical setting.
- Identify and implement solutions to overcome financial barriers to implementing cancer immunotherapy in the clinical setting.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: 12/10/19-12/10/20
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer ImmunotherapyTM educational series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.75 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-983-H04-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hour.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Amy Schippers, PA-C: Nothing to Disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period 12/10/19 through 12/10/20 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for Practical Barriers in Cancer Immunotherapy Treatment.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Edge, Apple Safari, Firefox, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain a discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com
| | Original Course Date: December 10, 2019
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.75 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | This course is no longer available for continuing education credits. Further, this course has been replaced by Module 1 of the SITC Certificate in Cancer Immunotherapy program, Basic Immunology Concepts, as serving as one of the pre-program courses for the ACI Live Virtual events. If you've registered for an ACI Live Virtual event you should have received a coupon code to take Basic Immunology Concepts for free.
Course Description
This interactive course provides an overview of the immune system, focusing on the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The foundational knowledge presented in this course is critical to understanding how the immune system can be harnessed to treat cancer through immunotherapy.

Target Audience
This activity is intended for physicians, pharmacists, registered nurses, advanced practice registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Christian M. Capitini, MD
Assistant Professor
University of Wisconsin Carbone Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the function of the components of the immune system, including relevant cells, molecules and organs of the immune system.
- Differentiate between the adaptive immune system and the innate immune system.
- Recognize the barriers to effective immunotherapy, including mechanisms by which tumors locally disable and/or evade the immune system.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: October 9, 2019 - October 9, 2020
Estimated time to complete activity: 1 hour
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
The 2019-2020 Advances in Cancer Immunotherapy™ series is supported, in part, by independent medical education grants from Amgen, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, Exelixis, Inc., Genentech, Incyte Corporation and Merck & Co., Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.

Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.0 contact hour (0.10 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-884-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.0 contact hour. Designated for 0.5 pharmacotherapy contact hour for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Christian Capitini, MD: Nothing to disclose
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME/CE credit for this activity. During the period October 9, 2019 through October 9, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please follow the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for "Introduction to Immunology – Third Edition."
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Successfully complete both the post-test with passing score of 80% or better and activity evaluation.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: October 09, 2019
|
Approved Credit: ACPE: 1 hour Contact HourANCC: 1 hour Contact HourACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 Credit
| MORE INFO |
 | Access the updated course, Immunotherapy for the Treatment of Hematologic Malignancies.
Immunotherapy for the Treatment of Hematologic Malignancies, is a CME-, CPE-, CNE- and MOC-certified activity.
Please note that continuing education credit for 2019 Interactive Course: Hematologic Malignancies and Immunotherapy (Advances in Cancer Immunotherapy™) is only available through May 3, 2019.
Course Description
This interactive course provides an overview of the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The course also provides the latest in immunotherapy treatment for hematological cancers, and summarizes many of the key findings that have resulted in recent FDA approvals for this kind of treatment. After completing this course, learners will gain an understanding of common approaches to cancer immunotherapy; how to implement cancer immunotherapy treatment for hematologic cancers and how to identify and manage common side effects of immunotherapy agents.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Naval Daver, MD
Associate Professor
University of Texasw MD Anderson Cancer Center
and
Christian Capitini, MD
Assistant Professor
University of Wisconsin - Madison Carbone Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatment for hematologic cancers.
- Identify the appropriate clinical management of common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: May 3, 2019 - May 3, 2020
Approximate Time to Complete: 30 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-607-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.2 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Naval Daver, MD - Consulting Fees - Pfizer, Otsaka, BMS, Daiichi-SanKyo, Jazz, Abbvie, Novartis and Contracted Research - Pfizer, BMS, Incyte,Sunesis, Karyopharm, Daiichi-SanKyo, Servier, Genentech, Abbvie, Glycomimetics
Christian Capitini, MD - Consulting Fee - Nektar Therapeutics
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Tara Withington, CAE, Executive Director of SITC, has an ownership interest as a partner at Executive Director, Inc.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 30, 2019 through April 30, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for 2019 Interactive Course: Hematological Malignancies and Immunotherapy (Advances in Cancer Immunotherapy™)
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussions of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: May 03, 2019
|
Approved Credit: ACPE: 0.50 hours CEUANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Access the updated course, Immunotherapy for the Treatment of Skin Cancers.
Immunotherapy for the Treatment of Skin Cancers, is a CME-, CPE-, CNE- and MOC-certified activity.
Course Description
This interactive course provides an overview of the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The course also provides the latest in immunotherapy treatment for melanoma and summarizes many of the key findings that have resulted in recent FDA approvals for this kind of treatment. After completing this course, learners will gain an understanding of common approaches to cancer immunotherapy; how to implement cancer immunotherapy treatment for melanoma and how to identify and manage common side effects of immunotherapy agents.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Isabella Glitza, MD, PhD
Oncologist
University of Texas MD Anderson Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatment for melanoma.
- Identify the appropriate clinical management of common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: May 1, 2019 - May 1, 2020
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
 
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-606-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.5 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Isabella Glitza, MD, PhD - Research - Bristol Myers Squib
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose. Tara Withington, CAE, Executive Director of SITC, has an ownership interest as a partner at Executive Director, Inc.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 24, 2019 through April 24, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for 2019 Interactive Course: Melanoma and Immunotherapy (Advances in Cancer Immunotherapy™)
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: May 01, 2019
|
Approved Credit: ACPE: 0.75 hours Contact HourANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Access Course
 Experts discuss the evolving role of checkpoint inhibition in patients with advanced bladder cancer. Experts discuss the evolving role of checkpoint inhibition in patients with advanced bladder cancer.
This activity is intended for oncologists, urologists, and other healthcare professionals who treat patients with bladder cancer.
The goal of this activity is to discuss the role of immunotherapy, including how to determine eligibility and how to manage immune-related adverse events (irAEs), in the treatment of advanced bladder cancer.
Approximate Time to Complete: 30 minutes
Credit Available: Apr. 30, 2019- Apr. 30, 2020
Developed through a partnership between SITC and Medscape.
| | Original Course Date: April 30, 2019
|
Approved Credit: ABIM: 0.50 hours ABIM MOC Part 2 CreditsACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | Access Course
 Nursing experts discuss the emergence of immunotherapy in GI cancers, focusing on the role of biomarkers and the management of irAEs. Nursing experts discuss the emergence of immunotherapy in GI cancers, focusing on the role of biomarkers and the management of irAEs.
This activity is intended for nurses and other healthcare professionals who manage patients with gastrointestinal cancers.
The goal of this activity is to discuss the emerging role of immunotherapy using immune checkpoint inhibitors in patients with gastrointestinal cancers.
Approximate Time to Complete: 30 minutes
Credit Available: Apr. 30, 2019 - Apr. 30, 2020
Developed through a partnership between SITC and Medscape.
| | Original Course Date: April 30, 2019
|
Approved Credit: ANCC: 0.50 hours Contact Hour
| MORE INFO |
 | Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Immunotherapy for the Treatment of Head and Neck Cancer module covers clinical data on the efficacy of approved therapies, the mechanism of action of approved therapies, patient selection for approved therapies, and dosing and sequencing of approved therapies for the treatment of head and neck cancers. The module also includes case studies on immunotherapy for the treatment of head and neck cancers.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Glenn J. Hanna, MD
Medical Oncologist, Dana-Farber Cancer Institute
Dana-Farber Cancer Institute
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy in treatment of head and neck cancer patients
- Identify the appropriate clinical management of common side effects of immunotherapy agents
- Implement cancer immunotherapy treatments for Head and Neck cancers
Continuing Education Information
Approximate Time to Complete: One hour and 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.75 contact hour(s) (0.175 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-730-H04-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.7 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Glenn J. Hanna, MD: Institutional support from BMS and EMD Serono
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: April 25, 2019
|
Approved Credit: ACPE: 1.75 hours CEUANCC: 1.70 hours Contact HourACCME (MD/DO): 1.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Access the updated course, Immunotherapy for the Treatment of Genitourinary Malignancies
Immunotherapy for the Treatment of Genitourinary Malignancies, is a CME-, CPE-, CNE- and MOC-certified activity.
Course Description
This interactive course provides an overview of the mechanisms by which the immune system eliminates foreign pathogens and cancer cells. The course also provides the latest in immunotherapy treatment for genitourinary cancer, including kidney, bladder and prostate cancers, and summarizes many of the key findings that have resulted in recent FDA approvals for this kind of treatment. After completing this course, learners will gain an understanding of common approaches to cancer immunotherapy; how to implement cancer immunotherapy treatment for genitourinary cancer (kidney, bladder and prostate cancers), and how to identify and manage common side effects of immunotherapy agents.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Lauren Harshman, MD
Assistant Professor and Co-director, Kidney Cancer Program
Dana-Farber Cancer Institute
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Implement cancer immunotherapy treatment for genitourinary cancers.
- Identify the appropriate clinical management of common side effects of immunotherapy agents.
SITC Online Education Disclaimer
A qualified healthcare professional should be consulted before using any therapeutic product discussed. Readers should verify all information and data before treating patients or employing any therapies described in this educational activity.
Continuing Education Information
Credit Available: March 28, 2019 - March 28, 2020
Approximate Time to Complete: 45 minutes
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of .75 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-608-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.4 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of commercial interest.
Faculty
Lauren Harshman, MD - Advisory/Research to Bayer, Exelixis, Genentech, Dendreon/Valient, Pfizer, Medivation/Astellas, Kew Group, Theragene, Corvus, Merck, Bristol-Meyers Squib, Jannsen, Sotio, Takeda, Sanofi, Novartis, Applied Clinical Education, PER
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Tara Withington, CAE, Executive Director of SITC, has an ownership interest as a partner at Executive Director, Inc.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period March 28, 2019 through March 28, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for 2019 Interactive Course: Genitourinary Cancer and Immunotherapy (Advances in Cancer Immunotherapy™)
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussions of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: March 28, 2019
|
Approved Credit: ACPE: 0.75 hours CEUANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Immunotherapy for the Treatment of Lung Cancer module covers clinical data on the efficacy of approved therapies, the mechanism of action of approved therapies, patient selection for approved therapies, and dosing and sequencing of approved therapies for the treatment of lung cancer. The module also includes case studies on immunotherapy for the treatment of lung cancer.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Deepa Rangachari, MD
Instructor, Medicine, Harvard Medical School
Beth Israel Deaconess Medical Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
-
Discuss the clinical data on the efficacy and mechanism of action of approved therapies
-
Recognize patient selection criteria for approved therapies
-
Describe appropriate dosing and sequencing therapies
-
Identify the appropriate clinical management of common side effects of immunotherapy agents
-
Implement cancer immunotherapy for the treatment of lung cancers
-
Describe the rationale for common approaches to cancer immunotherapy in treatment of naive NSCLC patients
-
Explain the role of biomarkers associated with response or lack of response to immunotherapy including PD-L1, tumor mutational burden (TMB), and epidermal growth factor receptor (EGFR)
Continuing Education Information
Approximate Time to Complete: 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-728-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.4 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Deepa Rangachari, MD: No conflict of interest to report.
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 0.75 hours CEUANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
 Exemplary video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplary video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Immunotherapy for the Treatment of Melanoma module covers clinical data on the efficacy of approved therapies, the mechanism of action of approved therapies, patient selection for approved therapies, and dosing and sequencing of approved therapies for the treatment of melanoma. The module also includes case studies on immunotherapy for the treatment of melanoma.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Elizabeth Buchbinder, MD
Instructor, Medicine, Dana-Farber Cancer Institute
Dana-Farber Cancer Institute
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy in treatment of melanoma patients
- Identify the appropriate clinical management of common side effects of immunotherapy agents
- Implement cancer immunotherapy treatments for melanoma
- Describe clinical efficacy of approved immunotherapies
- Recognize patient selection criteria for different immunotherapies
- Identify factors influencing dosing and choice of immunotherapy relative to other therapies for melanoma
Continuing Education Information
Approximate Time to Complete: 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-729-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.6 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Elizabeth Buchbinder, MD: Consulting Fees - Array Pharmaceuticals; Salary - Sanofi
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 0.75 hours CEUANCC: 0.60 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The What's Next for Cancer Immunotherapy module covers the future of cancer immunotherapy such as understanding important trends, new developments, potential approvals and disease states with promising data.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Christian Capitini, MD
Assistant Professor, Medicine
University of Wisconsin Carbone Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy.
- Become familiar with clinical data on the efficacy and mechanism of action of approved therapies.
Continuing Education Information
Approximate Time to Complete: 30 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.5 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.5 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-734-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.5 contact hours. Designated for 0.2 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Christian Capitini, MD: Consulting Fees - Nektar Therapeutics
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 0.50 hours CEUANCC: 0.50 hours Contact HourACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Immunotherapy for the Treatment of Hematologic Malignancies module covers clinical data on the efficacy of approved therapies, the mechanism of action of approved therapies, patient selection for approved therapies, and dosing and sequencing of approved therapies for the treatment of hematologic malignancies. The module also includes case studies on immunotherapy for the treatment of hematologic malignancies.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Nirav Shah, MD
Assistant Instructor, Medicine
Medical College of Wisconsin
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy for hematologic malignancies
- Identify the appropriate clinical management of common side effects of immunotherapy agents
- Implement cancer immunotherapy for Hematologic Malignancies
- Become familiar with clinical data on the efficacy and mechanism of action of approved therapies
- Recognize patient selection criteria for approved therapies
- Select appropriate dosing and sequencing of approved therapies
Continuing Education Information
Approximate Time to Complete: One hour and 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.75 contact hour(s) (0.175 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-731-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.7 contact hours. Designated for 0.5 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Nirav Shah, MD: Consulting fees - Juno Therapeutics, Contracted Research - Lentigen Technology, Ownership Interest - Exelexis, Geron, Oncosec
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 1.75 hours CEUANCC: 1.70 hours Contact HourACCME (MD/DO): 1.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Access the updated course, Practical Barriers in Cancer Immunotherapy Treatment.
Practical Barriers in Cancer Immunotherapy Treatment, is a CME-, CPE-, CNE- and MOC-certified activity.
Please note that continuing education credit for 2019 Video Series: Practical Barriers in Cancer Immunotherapy (Advances in Cancer ImmunotherapyTM) is only available through April 25, 2020.
Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Practical Barriers in Cancer Immunotherapy module covers practical barriers to implementing immunotherapy in a community setting (operations and reimbursement).. The module also includes a panel discussion in which experts in the field discuss common challenges they have faced including coverage and reimbursement challenges and adverse event managment.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Ryan Weight, DO, MD
Assistant Professor
University of Colorado
Monique Giordana, PharmD, BCOP
Hematology/Oncology Clinical Pharmacy Specialist
Regions Hospital
Lisa A. Kottschade, APRN, MSN, CNP
Associate Professor of Oncology/Nurse Practitioner
Mayo Clinic
Jodi Nordberg
Billing Operations Manager
UM Physicians
Sara Smith, PharmD, BCOP
Oncology Clinical Pharmacist
University of Minnesota Heath Maple Grove
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Identify solutions to overcome operational and financial barriers to integrating immunotherapy into their practice setting.
Continuing Education Information
Approximate Time to Complete: One hour and 30 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 1.5 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-733-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 1.5 contact hours. Designated for 0.2 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Ryan Weight, DO, MD: Consulting Fees - ACCC, Castle Biosciences, Novartis; Fees for Non-CME.CE Services - Merck; Contracted Research - BMS
Monique Giordana, PharmD, BCOP: No conflict of interest to report.
Lisa Kottschade, APRN, MSN, CNP: Consulting Fees - BMS, Array BioPharma
Jodi Nordberg: Fees for Non-CME/CE Services - Genentech
Sara Smith, PharmD, BCOP: Consulting Fees - Takeda
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 1.50 hours CEUANCC: 1.50 hours Contact HourACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
 Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Basic Prinicples of Cancer Immunotherapy module covers the basic principles and mechanisms behind cancer immunotherapy.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Theodore F. Logan, MD
Associate Professor, Medicine
Indiana University Simon Cancer Center
Learning Objectives
At the conclusion of this activy, the participants should be able to:
- Describe the rationale for common approaches to cancer immunotherapy
-
Recognize various approaches to cancer immunotherapy including therapeutic vaccine, adoptive cell transfer, cytokines, checkpoint inhibitor modulation, effector antibodies and antibody-drug conjugates
-
Explain the mechanism by which tumors evade the immune system and mechanisms to overcome this immunosuppression
-
List important biomarkers for measuring the immune response
Continuing Education Information
Approximate Time to Complete: 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement
 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-727-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.1 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Theodore F. Logan, MD: Consulting - Prometheus Laboratories; Contracted Research - Abbott Laboratories, Abraxix BioSciences, Acceleron Pharma, Amgen, Argos Therapeutics, AstraZeneca, AVEO, BioVex, Breistol-Myers Squibb, Eisai, Lilly, GlaxoSmithKline, Roche, Immatics, Merck, Novartis, Pfizer, Synta, Threshold Pharmaceuticals, Mellennium, TRACON Pharma, Cerulean Pharma, EMD Serono, Prometheus Laboratories, Macrogenics, Peloton Therapeutics, Iovance Biotherapeutics, MedImmune, Dynavax
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 25, 2019 through April 25, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2019
|
Approved Credit: ACPE: 0.75 hours CEUANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Access Course
 Apply best practices to monitoring for, diagnose, and manage potential irAEs in patient with cancer receiving ICIs Apply best practices to monitoring for, diagnose, and manage potential irAEs in patient with cancer receiving ICIs
This activity is intended for hematology/oncology specialists, emergency medicine physicians, and primary care physicians.
The goal of this activity is to build physician confidence in monitoring for, diagnosing, and managing immune-related adverse events (irAEs) that may occur in patients receiving immune checkpoint inhibitors (ICIs).
Approximate Time to Complete: 1 hour
Credit Available: Jan. 16, 2019 - Jan. 16, 2020
Developed through a partnership between SITC and Medscape.
| | Original Course Date: January 16, 2019
|
Approved Credit: ABIM: 1 hour ABIM MOC Part 2 CreditsACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™
| MORE INFO |
 | Course Description

Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
The online modules will facilitate understanding of the clinical applications of cancer immunotherapy for disease states with FDA-approved treatments, strategies for overcoming operational and reimbursement barriers to implementing immunotherapy in a community setting and the identification and management of immune-related adverse events.
The Immunotherapy for the Treatment of Genitourinary Malignancies module covers clinical data on the efficacy of approved therapies, the mechanism of action of approved therapies, patient selection for approved therapies, and dosing and sequencing of approved therapies for the treatment of Genitourinary Malignancies. The module also includes case studies on immunotherapy for the treatment of Genitourinary Malignancies.
Target Audience
This activity is intended for physicians, pharmacists, registered nurses and other healthcare professionals engaged in the care of patients with cancer.
Faculty
Lenard J. Appleman, MD, PhD
Associate Professor of Medicine
UPMC Hillman Cancer Center
Learning Objectives
At the conclusion of this activity, the participant should be able to:
- Describe the rationale for common approaches to cancer immunotherapy for genitourinary malignancies
- Identify the appropriate clinical management of common side effects of immunotherapy agents
- Implement cancer immunotherapy for genitourinary malignancies
- Become familiar with clincial data on the efficacy of approved therapies
- Recognize patient selection criteria for approved therapies
- Select appropriate dosing and sequencing of approved therapies (including timing and combination approaches)
Continuing Education Information
Approximate Time to Complete: 45 minutes
Credit Available: April 25, 2019 - April 25, 2020
Jointly provided by Postgraduate Institute for Medicine and the Society for Immunotherapy of Cancer.
This activity is supported, in part, by independent medical education grants from AbbVie Inc., Amgen Inc., AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb, Celgene Corporation, EMD Serono, Inc. and Pfizer Inc., Genentech, Incyte Corporation, Lilly USA, LLC, Merck & Co., Inc., Novartis Pharmaceuticals Corporation, Pfizer Inc. and Prometheus Laboratories Inc.
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Medical Education
The Postgraduate Institute for Medicine designates this enduring material for a maximum of 0.75 "AMA PRA Category 1 Credit(s)"™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Pharmacy Education
Postgraduate Institute for Medicine designates this continuing education activity for 0.75 contact hour(s) (0.075 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008162-9999-19-732-H01-P)
Type of Activity: Knowledge
Continuing Nursing Education
The maximum number of hours awarded for this Continuing Nursing Education activity is 0.7 contact hours. Designated for 0.3 pharmacotherapy contact hours for Advanced Practice Registered Nurses.
Disclosure of Conflicts of Interest
Postgraduate Institute for Medicine (PIM) requires instructors, planners, managers and other individuals who are in a position to control the content of this activity to disclose any real or apparent conflict of interest (COI) they may have as related to the content of this activity. All identified COI are thoroughly vetted and resolved according to PIM policy. PIM is committed to providing its learners with high quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
Faculty
Leonard J. Appleman, MD, PhD: Contracted Research - Astellas, BMS, Bayer, Exolius, Merck, Roche
Planners and Managers
The PIM planners and managers have nothing to disclose. The Society for Immunotherapy of Cancer planners and managers have nothing to disclose.
Method of Participation and Request for Credit
There are no fees for participating and receiving CME credit for this activity. During the period April 17, 2019 through April 17, 2020 participants must read the learning objectives and faculty disclosures and study the educational activity.
If you wish to receive acknowledgment for completing this activity, please following the steps below:
- After completing the course, click My Credit from the left-hand side of the SITC connectED homepage.
- Under Pending Credit, click Request Credit for the appropriate course title.
- Ensure that the appropriate Credit Reporting Organization is selected. Click Continue.
- Complete the post-test and survey.
- Click Process Credit.
- Your Certificate will be available to view in the Submitted Credit section.
For Pharmacists: Upon successfully completing the post-test with a score of 75% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4-5 weeks.
Media
Internet
Hardware and Software Requirements
SITC connectED requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Contact PIM: www.pimed.com.
| | Original Course Date: January 31, 2018
|
Approved Credit: ACPE: 0.75 hours CEUANCC: 0.70 hours Contact HourACCME (MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.75 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation
| MORE INFO |
|
Certificate in Cancer Immunotherapy
Certificate program for licensed physicians (U.S. licensed MD / DO or global equivalent) and practicing, licensed NPs, PAs, PharmDs (or RPh degree holders involved in direct clinical services), or global equivalent, interested in achieving an identifiable designation (SITC-G; G = graduate) for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. The program is also available to others, but they will not be eligible to earn the certificate. |
 | 
The Society for Immunotherapy of Cancer (SITC) is pleased to offer physicians and qualified healthcare providers the opportunity to earn a Certificate in Cancer Immunotherapy, supporting their knowledge and skills to provide effective and safe care for patients treated with cancer immunotherapy. Learners can expect to develop a comprehensive understanding of cancer immunotherapies, including checkpoint inhibitors, cell therapies and many others, as well as the management of side effects, allowing them to successfully implement immunotherapy in clinical practice.
The Certificate in Cancer Immunotherapy consists of eight learning modules and a final assessment. You will earn SITC Graduate in Cancer Immunotherapy (SITC-G) certificate upon successfully completing all the 8 modules and qualfying in the final assessment. The SITC-G certificate identifies a healthcare provider as completing specialized training in cancer immunotherapy. All modules are approved for CME, CNE, CPE and MOC credits, except where noted.
SITC Graduate in Cancer Immunotherapy / SITC-G

Cost of certification
- SITC Member Price: $240
- Non-Member Price: $320
- SITC Member Price for Low to Lower-Middle Income Economies: $0*
*Note: This is not available to those in the industry work setting.
Click here for the List of Low to Lower-Middle Income Economies
Target Audiences
The primary target audience for the program is treating physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate will also be available to practicing, licensed NPs, PAs, and PharmDs (or RPh degree holders involved in direct clinical services), or global equivalent. The courses will be available to those who are not practicing clinicians, but they will not be eligible to earn the certificate.
Requirements
The requirements to earn the certificate include:
- Must be a treating physician (U.S. licensed MD / DO or global equivalent), or a practicing licensed NP, PA, PharmD or RPh degree holder involved in direct clinical services, or global equivalent
- Complete the coursework (all eight modules)
- anticipated to be approximately 1 hour each, for a total of 8 hours
- Pass all end-of-module assessments
- Successfully complete a comprehensive exam covering all modules
Certificate Term
The certificate term is two years. Then, to earn a current certificate, which allows one to continue to use the SITC-G designation, an individual will need to take and pass updated coursework and assessments. If one misses the deadline for reissuance, he or she will need to retake and pass the full program.
Certificate Program Task Force
Chair
- Robert L. Ferris, MD, PhD – University of Pittsburgh Medical Center Hillman Cancer Center
Members
- Umar Farooq, MD – University of Iowa
- Silvia Formenti, MD – Weill Cornell Medicine
- Sigrun Hallmeyer, MD – Advocate Medical Group
- Jose Lutzky, MD, FACP – University of Miami Sylvester Cancer Center
- George Weiner, MD – University of Iowa
Requirements
The requirements to earn the certificate include:
• Complete the coursework (all eight modules)—anticipated to be approximately 1.5 hours each for a total of 12–16 hours
• Successfully complete a comprehensive exam covering all modules
Learner Notification
This program consists of eight modules. Continuing Education credit information for the modules can be found below, and at the module level. Please note, that each module counts for a maximum of 1.50 AMA PRA Category 1 Credits™ for physicians and 1.50 contact hours for nurses and 1.50 knowledge-based contact hours for pharmacists.
Continuing Education (CE) Language
Certificate in Cancer Immunotherapy Webseries Online
Physicians / Nurses / Pharmacists

In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Credit Designation Statement – Upon successful completion of all content in this program, Partners designatse this enduring material for a maximum of 8.5 AMA PRA Category 1 CreditsTM for physicians and 8.5 contact hours for nurses and 8.5 knowledge-based contact hours for pharmacists. Learners should claim only the credit commensurate with the extent of their participation in the activity.
NOTE to Pharmacists: The only official Statement of Credit is the one you pull from CPE Monitor. You must request your certificate within 30 days of the activity to meet the deadline for submission to CPE Monitor.
ABIM MOC Credit
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 8.5 Part 2 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program.
| | Original Course Date: December 06, 2022
On-Demand Release Date: Available Now | MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 1: Basic Immunology Concepts, will cover some of the key immunology needed to understand the rest of the certificate program.
- Original Release Date: August 3, 2020
- Expiration Date: June 1, 2027
- Date of Last Review: July 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Click on image for FREE PREVIEW
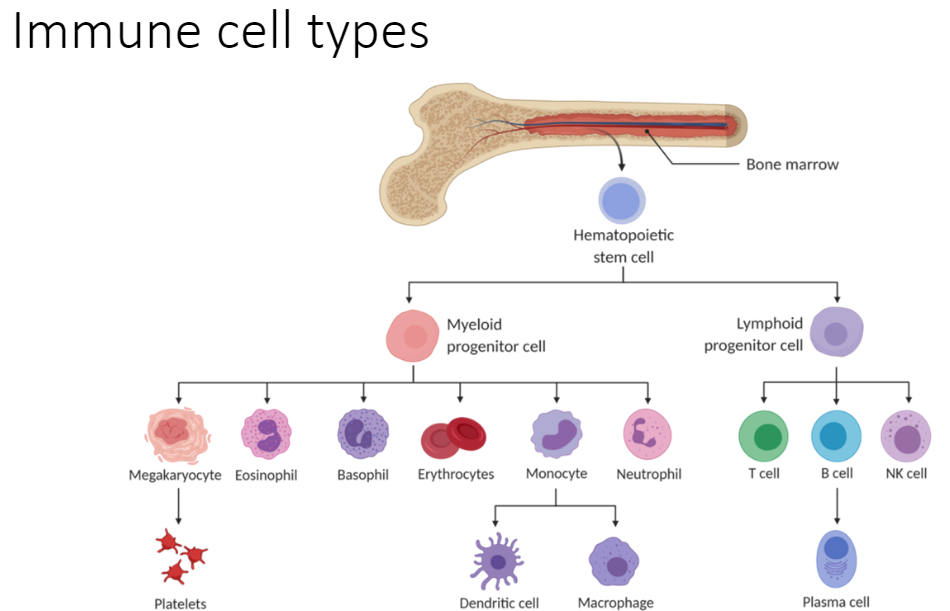
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
At the conclusion of this activity, the participant should be able to: |
| Basic Properties of the Immune System |
- Describe the differences between innate immunity and adaptive immunity.
- Describe the differences between humoral immunity and cellular immunity.
- Describe the concept of self/non-self discrimination and immunologic memory.
|
| Cells and Tissues of the Immune System |
- Distinguish between the basic cell types involved in the immune response and their function.
- Describe the architecture and functions of various immunologic tissues including the thymus, spleen, lymph nodes, and bone marrow.
|
| Innate Immunity |
- Describe the key components of innate immunity including pattern recognition receptors and cells of the innate immune system.
- Describe the function and regulation of innate immunity.
- Describe how activation of innate immunity influences adaptive immune response.
- Recognize how host cell death influences innate immunity.
|
| Adaptive Immunity |
- Describe the components of the adaptive immune system including both humoral and cellular immunity.
- Describe the function and regulation of adaptive immunity.
- Define immune tolerance.
- Describe how immune tolerance impacts adaptive immunity.
|
| Antigen Presentation |
- Distinguish class I and class II MHC antigen presentation.
- Describe antigen uptake, processing, and presentation.
- Describe the concept of cross-presentation.
|
| Effector Immune Responses |
- Identify the cell populations involved in effector immune responses.
- Describe the different mechanisms of effector functions.
- Describe how effector functions are regulated.
|
| Inhibitory and Activating Immune Regulation |
- Describe the functions of major suppressive cell populations (Treg, MDSC).
- Describe immunological synapses.
- Describe the molecular mechanisms of immune cell activation and inhibition.
- Distinguish between immune cell activation and inhibition of suppression as a pharmacological strategy.
|
| Integration of the Immune Response |
- Describe how the arms of the immune system interact.
- List the host and environmental factors that influence the immune response.
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD
Hillman Professor of Oncology
Director, UPMC Hillman Cancer Center
Associate Vice Chancellor for Cancer Research
University of Pittsburgh Medical Center, Pittsburgh, PAnderson Cancer Center, Houston, TX
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-177-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 6/1/2025 through 6/1/2027 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: August 03, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 1 hour Certificate of Participation: 1.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1.50 hours Contact HourANCC: 1.50 hours Contact Hour
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 2: Basic Cancer Immunotherapy Concepts, will cover the biological foundation of cancer immunotherapy, applying the basic immunology you learned in the last module to help lay the foundation for future topics in this program.
- Original Release Date: October 26, 2020
- Expiration Date: October 27, 2026
- Date of Last Review: October 2023
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Click on image for FREE PREVIEW

Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
At the conclusion of this activity, participant should be able to: |
| Basis of Tumor Immunosurveillance and Immunotherapy |
- Describe how the immune system recognizes and eliminates cancer cells, including the concept of immunologic memory.
- Characterize the function of specific immune cell populations in mediating tumor immunotherapy and/or immune suppression.
- Distinguish between immunosurveillance, immunoediting and immunotherapy of cancer.
- Identify the implications of immune depleted, excluded and infiltrated tumor microenvironments.
- Identify how both central and peripheral tolerance can impact immunosurveillance and immunotherapy.
- Describe the difference between primary and acquired resistance to immunotherapy.
- Apply concepts of the cancer-immunity cycle to identify how biomarkers inform and influence tumor immunotherapy.
|
| Immunologic Effects of Other Therapies |
- Describe the immunologic effects of standard cancer therapeutics on the tumor microenvironment and immune system.
- Describe the potential impact of other, non-cancer agents, on tumor immunotherapy responses and toxicities.
- Characterize the impact of standard cancer treatments on the efficacy of cancer immunotherapy.
- Characterize the impact of standard cancer treatments on the toxicity of cancer immunotherapy.
- Distinguish total body versus focal radiotherapy effects.
- Recognize the impact of intratumoral injection and other local therapies on the tumor microenvironment and anti-tumor immune response.
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Hussein Tawbi, MD, PhD
Deputy Chair and Professor, Department of Melanoma Medical Oncology
Director of Melanoma Clinical Research & Early Drug Development
Co-Director, MD Anderson Brain Metastasis Clinic
University of Texas MD Anderson Cancer Center, Houston, TX
|
- Consultant: Genentech, BMS, Novartis, Merck, Array
- Contracted Research: Genentech, BMS, Novartis, Merck, GSK
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-23-272-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 10/27/2023 through 10/27/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: October 26, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1.50 hours Contact HourANCC: 1.50 hours Contact Hour
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 3: Immune Checkpoint Blockade, will cover the biological foundation of immune checkpoint blockade therapies as well as diving into their clinical use.
- Original Release Date: December 1, 2022
- Expiration Date: January 8, 2027
- Date of Last Review: January 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Click on image for FREE PREVIEW
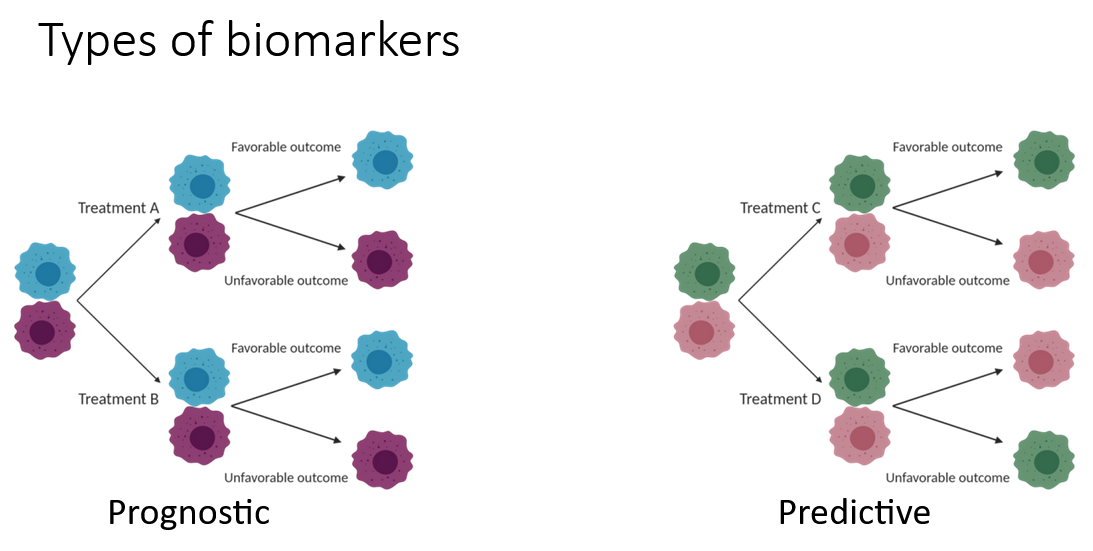
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
At the conclusion of this activity, participant should be able to: |
| Basic Mechanisms of Checkpoint Blockade in Immunotherapy |
- Describe the basis for co-stimulation in T cell activation and priming.
- Explain the mechanisms and consequences of co-inhibitory immune checkpoint receptors (ICR).
- Identify negative downstream regulatory motifs and pathways associated with ICR signaling.
- Distinguish the distinct activities of antibodies that target PD-1 compared to those that target PD-L1.
|
| Biomarkers of Response to ICB and General Clinical Utility |
- Describe major biomarkers for patient eligibility for the use of immune checkpoint blockade across cancers (PD-L1 expression and extent, IFN signature, CD8 infiltration, Tumor Mutational Burden) and their relative value for prediction of clinical benefit.
- Distinguish between validated and investigational biomarkers.
- Describe the use of biomarkers to predict ORR, PFS and OS.
- Identify patient-specific factors that may predict response (tumor burden, prior chemotherapy exposure, age, gender, site of disease, prior radiotherapy, etc.).
|
| Immune Checkpoint Inhibitors and Combination Approaches |
- Describe general clinical benefits of ICB with PD-1 or CTLA-4 monotherapy, or ICB combinations (including IO+IO and IO+other cancer therapy combinations).
- Distinguish the benefits, dose and schedules of combination ICB versus the benefits, dose and schedule of conventional therapies (chemotherapy, radiation therapy and targeted therapies).
- Identify validated vs investigational ICB combinations, and relevant cancer types.
|
| Monitoring Response to Checkpoint Blockade |
- Distinguish response criteria in ICB vs standard cancer treatment.
- Describe kinetics and patterns of response to ICB.
- Distinguish pseudoprogression from true disease progression.
- Identify when to suspend or discontinue ICB treatment.
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD
Hillman Professor of Oncology
Director, UPMC Hillman Cancer Center
Associate Vice Chancellor for Cancer Research
University of Pittsburgh Medical Center, Pittsburgh, PAnderson Cancer Center, Houston, TX
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-014-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 1/8/2024 through 1/8/2027 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: December 01, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1.50 hours Contact HourANCC: 1.50 hours Contact Hour
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 4: Managing Immune Checkpoint Inhibitor Adverse Events, will cover the presentations, diagnosis and management of adverse events resulting from immune checkpoint inhibitor treatments.
- Original Release Date: September 15, 2021
- Expiration Date: July 9, 2026
- Date of Last Review: June 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 60 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
Upon completion of this activity, participants should be able to: |
| Recognition, Frequency and Kinetics of Immune-Related Toxicities |
- Identify the immune-related toxicities associated with checkpoint inhibition.
- Classify severity of immune-related side effects.
- Describe the kinetics of immune-related toxicities of immune checkpoint inhibition.
- Distinguish immune-related side effects from those related to disease progression.
- Recognize and manage hyperprogression.
- Manage concurrent multiple adverse events.
- Manage immunosuppressive medications used to treat adverse events.
|
| Management of Immune-Related Colitis |
- Describe how to diagnose immune-related colitis.
- Describe the frequency and time to development of immune-related colitis associated with distinct immunotherapeutic drugs.
- Manage grade 1/2 colitis and steroid refractory immune-related colitis.
|
| Management of Immune-Related Pneumonitis |
- Diagnose immune-related pneumonitis.
- Describe the frequency of immune-related pneumonitis with distinct cancer types.
- Manage immune-related pneumonitis.
|
| Management of Immune-Related Endocrinopathies |
- List immune-related endocrinopathies.
- Recognize and diagnose immune-related endocrinopathies.
- Treat immune-related endocrinopathies based on the severity of the toxicity.
- Counsel patients about long-term consequences and management of endocrinopathies.
|
| Management of Immune-Related Hepatitis |
- Recognize and diagnose immune-related hepatitis.
- Treat immune-related hepatitis based on the severity of the toxicity.
|
| Management of Immune-Related Cutaneous Events |
- List immune-related cutaneous events.
- Recognize and diagnose immune-related cutaneous events.
- Treat immune-related cutaneous events based on the severity of the toxicity.
|
| Management of Other (Rare) Immune-related Adverse Events |
- List rare immune-related adverse events.
- Recognize and diagnose rare immune-related adverse events.
- Treat rare immune-related adverse events based on the severity of the toxicity.
|
| Unique Aspects of IO-Containing Combination Therapies |
- Describe the patterns and severity of adverse events with IO-containing combination therapies relative to the single agents.
- Distinguish between immune-related and other toxicities in patients receiving IO-containing combination therapies.
|
| Chronic Toxicities Associated with Cancer Immunotherapy |
- List chronic toxicities of immunotherapy.
- Describe the time course of chronic toxicities of immunotherapy.
- Manage the chronic toxicities of immunotherapy.
|
| Management of Non-Immune-Mediated Adverse Events |
- List non-immune-mediated adverse events.
- Recognize non-immune-mediated adverse events.
- Treat non-immune-mediated adverse events.
|
| Management of ICB Therapy in the Context of an Adverse Event |
- Describe when ICB therapy can be continued, reduced or discontinued in the face of toxicity.
- Describe when ICB therapy can be reinstated following management of toxicity.
|
Faculty and Disclosure of Conflicts of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Jason Luke, MD, FACP
Associate Professor of Medicine, Division of Hematology/Oncology
Director, Cancer Immunotherapeutics Center within the UPMC Hillman Cancer Immunology and Immunotherapy Program
University of Pittsburch Medical Center, Pittsburgh, PA
|
- Consultant: Fstar, RefleXion, Xilio Abbvie, Alnylam, Bayer, Bristol-Myers Squibb, Checkmate, Crown, Cstone, Eisai, EMD Serono, Flame, Genentech, Gilead, Kadmon, KSQ, Janssen, Immunocore, Inzen, Macrogenics, Merck, Mersana, Nektar, Novartis, Pfizer, Regeneron, Ribon, Rubius, Silicon, Synlogic, TRex, Werewolf, Xencor
- Contracted Research: AbbVie, Agios (IIT), Array (IIT), Astellas, Bristol-Myers Squibb (IIT & industry), Corvus, EMD Serono, Fstar, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Numab, Replimmune, Rubius, Spring bank, Synlogic, Takeda, Trishula, Tizona, Xencor
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.0 contact hours.
Pharmacy Continuing Education
Partners designates this continuing education activity for 1.0 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-160-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 7/9/2024 through 7/9/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: November 02, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditANCC: 1 hour Contact HourACPE: 1 hour Contact Hour
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 5: Other Approaches (Cytokines, Vaccines, and Immune Cell Engagers), will cover the biological foundation and clinical implementation of cytokines, vaccines and immune cell engagers as immunotherapies for cancer, including adverse event management.
- Original Release Date: July 5, 2021
- Expiration Date: July 9, 2026
- Date of Last Review: June 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 90 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
Upon completion of this activity, the participant should be able to: |
| Basic Mechanisms |
- Define the types and functions of cytokines and chemokines used in cancer immunotherapy.
- Distinguish among the various types of cancer vaccines.
- Describe the different types and functions of tumor antigens used in cancer vaccines.
- Classify the different delivery platforms for cancer vaccines.
- Describe how personalized cancer vaccines differ from standard cancer vaccines.
- Identify different methods and routes of administration of immunotherapy agents.
- Define the types of immunologic adjuvants and their distinct functions in promoting immune responses.
- Recognize the role of innate immune activators in promoting tumor immunotherapy.
- Describe the mechanism of action of immune cell engagers.
- Compare and contrast immune cell engagers with adoptive cellular therapies.
- Describe potential mechanisms of relapse following immune cell engager therapies.
|
| Clinical Use and Development |
- Appreciate the historical data on vaccine and cytokine treatment of malignancy.
- List current clinical indications for vaccines, cytokines, and immune cell engagers in cancer treatment.
- Explain how immunologic boosting can enhance anti-tumor immunity with vaccines.
- Describe the role of immunotherapy and vaccines in cancer prevention.
- Characterize new and emerging vaccines, cytokines and immune cell engagers under clinical investigation.
- Describe how cytokine levels may be used as prognostic and predictive biomarkers for cancer immunotherapy.
|
| Combination Approaches |
- Describe how cytokines, vaccines, and immune cell engagers can be combined with other immunotherapy agents to promote T cell recruitment and anti-tumor immunity.
- Recognize the role of non-immune agents in promoting tumor immunity in combination with cytokines, vaccines, and immune cell engagers.
- Define immunogenic cell death (ICD) and describe how combination approaches promote ICD and anti-tumor immunity.
- Explain the abscopal effect of local therapy.
- Describe how other agents can enhance immunotherapy.
|
| Patient Selection, Management and Monitoring |
- Apply criteria for patient selection for IL-2 or interferon therapy.
- Identify contraindications to treatment with cytokines.
- Describe methods needed to safely monitor patients on high-dose IL-2 therapy.
- Describe criteria for patient selection for vaccine treatment.
- Identify contraindications to treatment with vaccine therapy.
- Describe patient selection criteria for immune cell engager therapies.
- Identify contraindications to immune cell engager therapies.
- Determine duration of treatment and when to assess patients for clinical response.
|
| Management of Adverse Events |
- Recognize the type and severity of adverse events associated with cytokine, vaccine, and immune cell engager administration.
- Apply appropriate interventions to ameliorate toxicities associated with cytokines, vaccines, and immune cell engagers.
- Identify appropriate institutional and provider resources and procedures needed to manage cytokine-related toxicities.
- Recognize the importance of continuing education for high-dose cytokine delivery teams.
- Recognize the interactions between cytokines, vaccines, and immune cell engagers with other immunotherapy and non-immunotherapy cancer agents for causing unknown or unexpected adverse events.
|
Faculty and Disclosure of Conflicts of Interest
Partners for Advancing Clinical Education (Partners) requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Umar Farooq
Senior Vice President
Global Head of Cell Therapy Research
Universty of Iowa
|
- Consultant: Kite Pharma, Inc
- Researcher: Regeneron Pharmaceuticals; Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 1.5 contact hour(s) (0.15 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-161-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 7/9/2024 through 7/9/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: December 07, 2020
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1.50 hours Contact HourANCC: 1.50 hours Contact Hour
| MORE INFO |
 | Please note, this activity does not offer continuing education credit.
Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 6: Oncolytic Viruses and Local Therapy, will cover the biological foundation and clinical implementation of intratumoral therapies and oncolytic viruses.
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Faculty
Brian Gastman, MD
Professor, Department of Surgery, School of Medicine
Member, Hematopoietic and Immune Cancer Biology Program, Case Comprehensive Cancer Center
Case Western Reserve University, Cleveland, OH
Department of Plastic Surgery
Cleveland Clinic Main Campus, Cleveland, OH
Ann Silk, MD, MS
Co-Director of the Merkel Cell Carcinoma Center of Excellence
Dana-Farber Cancer Institute, Boston, MA
Educational Objectives
| Topic |
At the conclusion of this activity, the participant should be able to: |
| Basic Mechanisms of Oncolytic Viruses and Intralesional Therapy |
- Describe the rationale for intralesional therapy as a strategy for modulating tumor cells and promoting immunity.
- Distinguish the differences between oncolytic viruses and non-viral intralesional approaches.
- Classify the different viruses available for cancer immunotherapy and their advantages and disadvantages.
- List approved oncolytic viruses and intralesional agents.
- Describe the dual mechanism of action of oncolytic viruses in mediating anti-tumor immunity.
- Identify how oncolytic viruses can deliver transgenes to promote anti-tumor activity.
- Describe alternative delivery routes of administration for oncolytic viruses and other intralesional agents.
|
| Patient Selection and Monitoring for Oncolytic Virus Therapies and Intralesional Therapy |
- Identify eligible patients for oncolytic viruses and intralesional therapy.
- Determine when oncolytic viruses and/or intralesional therapy should be considered in patient management.
- Select appropriate tumors for injection of an oncolytic virus and/or intralesional therapy.
- Determine the volume of oncolytic virus and/or intralesional agent for patient therapy.
- Describe the process of injecting oncolytic viruses and/or intralesional treatments in patients with cancer.
- Identify the role of interventional radiology in administration of intralesional treatments to deep and/or visceral tumors.
- Apply appropriate injection site management following injection of intralesional agents.
- Define the patterns of response with oncolytic viruses and other intralesional treatments.
- Identify contraindications to oncolytic virus and/or intralesional therapy.
- Outline the potential role for oncolytic viruses and intralesional therapy as part of combination regimens for patients with cancer.
|
| Management of Oncolytic Virus and Intralesional Therapy Adverse Events |
- Identify the most common adverse events seen with oncolytic viruses and/or other intralesional treatments.
- Prescribe appropriate intervention for managing common adverse events associated with oncolytic viruses and intralesional therapies.
|
| Logistical and Biosafety Issues Associated with Oncolytic Virus Therapy |
- Describe the biosafety issues associated with oncolytic virus administration.
- Recognize the potential for bioshedding and contact transmission associated with oncolytic viruses.
- Explain how to modify patient flow to optimize integration of oncolytic viruses and intralesional treatments into clinical practice.
- Implement standard operating procedures for safe delivery of oncolytic viruses and intralesional treatments into clinical practice.
|
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion.
| | Original Course Date: January 04, 2021
On-Demand Release Date: Available Now |
Approved Credit: CoP: 0.00 hours Certificate of Participation
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 7: CAR T Cell and Cellular Therapy, will cover the mechanisms and clinical administration of cellular therapies for cancer, including an emphasis on adverse event management.
- Original Release Date: December 1, 2021
- Expiration Date: April 1, 2026
- Date of Last Review: March 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 30 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
Upon completion of this activity, participants should be able to: |
| Basic Mechanisms of Adoptive T Cell Immunotherapy |
- Describe the biologic basis for adoptive T cell therapy of cancer.
- Define significance of different target antigens and signaling moieties in CAR T cell activity.
- Contrast the differences between CAR T and other adoptive T cell strategies.
- Distinguish between different CAR T preparations such as target antigen but also viral vector culture conditions and signaling moieties.
|
| Cellular Therapies and Current Clinical Indications |
- Classify the types of adoptive T cell therapies approved for cancer treatment.
- Describe clinical indications of approved therapies.
- Describe off-label uses of CAR T cell therapy.
|
| Patient Selection for T Cell-based Therapy |
- Identify when patients should be referred for cellular therapy.
- Recognize contraindications to adoptive cell administration.
- Describe the timeline of adoptive cellular therapy preparation and administration.
|
| Management of CAR T Cell Administration and Adverse Events |
- Describe the preparation of CAR T and patient disease management prior to administration.
- Recognize the different types and patterns of CAR T-related toxicity.
- Outline approaches to monitoring patients for CAR T-related toxicity including cytokine release syndrome and neurotoxicity.
- Describe grading and management of cytokine release syndrome and neurotoxicity.
- Describe typical kinetics of response of malignancy to CAR T cell therapy.
- Describe long-term monitoring and management of CAR T cell patients including identification and management of long-term toxicities such as cytopenias and immunodeficiency.
- Describe the resources required for outpatient management of CAR T cell therapy.
|
| Requirements for CAR T Cell Program Development |
- Describe the requirements for site certification for administering cellular therapies, including REMS training.
- Identify other medical specialists who need to participate in REMS training and care of CAR T patients.
- Outline data reporting requirements for CAR T cell programs.
- Describe inpatient versus outpatient management and monitoring of CAR T cell patients.
|
Faculty and Disclosure of Conflicts of Interest
Partners for Advancing Clinical Education (Partners) requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Carl June, MD
Richard W. Vague Professor In Immunotherapy, Department of Pathology and Laboratory Medicine
Director, Parker Institute for Cancer Immunotherapy
Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA
|
- Novartis: Royalties; AC Immune, Bluesphere Bio, Cellares, Celldex, Cabaletta, Carisma, DeCART, Kiadis, Poseida, Viracta, WIRB-Copernicus Group: Consulting
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 0.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 0.5 contact hours.
Pharmacy Continuing Education
PACE designates this continuing education activity for 0.5 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-081-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 4/1/2024 through 4/1/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: February 01, 2021
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 0.50 hours AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 0.50 hours AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 0.50 hours Contact HourANCC: 0.50 hours Contact Hour
| MORE INFO |
 | Course Description
This interactive online course is part of the Certificate in Cancer Immunotherapy program, produced by the Society for Immunotherapy of Cancer (SITC). The purpose of the certificate program is to provide hospitals, medical centers, third-party payers, referring physicians, trainees and patients with an identifiable designation for healthcare providers who can safely and effectively participate in administration of immunotherapies and manage patients treated with these approaches. View all eight modules of the program and a detailed description of the program here.
This course, Module 8: Implementing Cancer Immunotherapy in Clinical Practice, will cover the practical aspects of instituting immunotherapy treatments in a clinical practice, such as education considerations, value-based care, and access to patient and provider resources.
- Original Release Date: May 2, 2022
- Expiration Date: May 2, 2026
- Date of Last Review: April 2024
- Jointly provided by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC)
- Estimated time to complete the activity: 60 minutes
- For additional information about the accreditation of this activity, please visit https://partnersed.com
- Computer system hardware/software requirements: SITC connectED requires a modern web browser (Edge, Apple Safari, Google Chrome, Internet Explorer 7+) and the ability to listen to audio with the content.
Target Audience
The program is available to licensed physicians (U.S. licensed MD / DO or global equivalent). The courses and earning of the certificate (SITC-G; G = graduate) are also available to practicing, licensed NPs, PAs, and PharmDs or global equivalent. RPh degree holders are eligible if they are involved in direct clinical services. The courses are available to others who are not practicing clinicians, but they will not be eligible to earn the certificate.
Educational Objectives
| Topic |
Upon completion of this activity, participants should be able to: |
| How Cancer Immunotherapy Integrates into Value-based Clinical Care |
- Contrast the role of IO versus standard cancer therapies in the setting of personalized care approaches.
- Evaluate IO in the NCCN/ASCO value framework.
- Interpret published data on Patient Reported Outcomes.
- Analyze cost of IO in the setting of quality of life, earning potential preserved, duration of response, and duration of therapy.
|
| Optimizing Resources for Cancer Immunotherapy Patient Care |
- Identify and access local, regional and national support foundations and financial support programs.
- Identify patient education resources.
- Tailor patient and staff communication to the dynamic and complex nature of IO, including continuous education strategies, telemedicine, etc.
|
| Educating Non-cancer Professionals about Cancer Immunotherapy |
- Build a dedicated multidisciplinary team with expertise in immuno-oncology administration, adverse events diagnosis and management.
- Develop subspecialty-targeted learning programs for education on IO.
- Identify best practices of communicating with primary care physicians and emergency physicians on how to recognize treatment-related adverse events.
|
| Patient Selection in Immunotherapy |
- Interpret the value and limitations of current biomarkers in different IO settings.
- Distinguish the risk/benefit ratios for each individual case based on published data, expert opinion or personal experience.
- Describe the key elements of a detailed informed consent discussion.
- Describe IO therapy in populations traditionally deemed ineligible for clinical trials.
|
Faculty and Disclosure of Conflict of Interest
Partners requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to PACE policies. Others involved in the planning of this activity have no relevant financial relationships.
| Presenting Faculty |
Conflict of Interest |
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
- Consultant: Cardinal Health
|
| Certificate Program Task Force |
Conflict of Interest |
|
Robert L. Ferris, MD, PhD (Chair)
UPMC Hillman Cancer Center
|
- Researcher: Astra-Zeneca/Medimmune, Bristol Myers Squibb, Merck, Novasenta, Tesaro
- Consultant/Advisor/Speaker: Achilles Therapeutics; Adagene Incorporated, Adaptimmune, Aduro Biotech Inc, Astra-Zeneca/MedImmune; Bicara Therapeutics, Inc, Bristol-Myers Squibb, Brooklyn Immunotherapeutic, Cantenion, Coherus BioSciences, Inc, CureVac, Cytoagents, Eisai Europe Limited, EMD Serono, Everest Clinical Research Corporation, F. Hoffman-La Roche Ltd., Federation Bio, Inc, Genmab, Genocea Biosciences, Inc, Hookipa Biotech GmbH, Instill Bio, Inc, Kowa Research Institute, Inc, Lifescience Dynamics Limited, MacroGenics, Inc, MeiraGTx, LLC, Merck, Merus N.V, Mirati Therapeutics, Inc, Mirror Biologics Inc, Nanobiotix, Novartis Pharmaceutical Corporation, Novasenta, Numab Therapeutics AG, OncoCyte Corporation, Pfizer, PPD Development, L.P., Rakuten Medical, Inc, Regeneron, Sanofi, Seagen, Inc, SIRPant Immunotherapeutics, Inc, Tesaro, Vir Biotechnology, Inc, Zymeworks Inc
- Stock holder: Novasenta
|
|
Umar Farooq, MD
University of Iowa
|
- Researcher: Regeneron Pharmaceuticals
- Consultant/Advisor/Speaker: Immpact Bio, Caribou Biosciences, Kite Pharma, MoprhoSys
|
|
Silvia Formenti, MD
Weill Cornell Medicine
|
- Grant/Research Support: Bristol Myers Squibb, Varian, Regeneron, Merck, Celldex, ViewRay, AstraZeneca
- Consultant/Honoraria: Bayer, Bristol Myers Squibb, Varian, ViewRay, Elekta, Janssen, Regeneron, GlaxoSmithKline, Eisai, Astra Zeneca, MedImmune, Merck US, EMD Serono/Merck, Genentech/ROCHE, Nanobiotix
|
|
Sigrun Hallmeyer, MD
Advocate Medical Group
|
|
|
Jose Lutzky, MD, FACP
University of Miami Sylvester Cancer Center
|
- Researcher: BMS
- Consultant/Advisor/Speaker: Castle, Iovance, Vyriad, Replimune, Takeda, Oncotelic, T-Nanobio, Agenus, Celldex
- Independent Contractor: BMS, Novartis, Iovance, Replimune, Regeneron, InstilBio, Syntrix, BioNtech, Foghorn, Trisalus, Agenus, Inmatics, Takeda, Dragonfly
|
|
George Weiner, MD
University of Iowa
|
- Researcher: Regeneron, Pfizer
|
Joint Accreditation Statement
Physicians / Nurses / Pharmacists
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
American Board of Internal Medicine (ABIM) Maintenance of Certification
 Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
To receive CME credit and/or MOC points, you MUST pass the posttest and complete the evaluation. For ABIM MOC points, your information will be shared with the ABIM through PACE’s Joint Accreditation Program and Activity Reporting System (JAPARS). Please allow 6-8 weeks for your MOC points to appear on your ABIM records. By sharing your Diplomate Board ID # and DOB, you are giving PACE permission to use this information/data to report your participation to these Boards JA-PARS.
Nursing Continuing Professional Development
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 1.0 contact hours.
Pharmacy Continuing Education
Partners designates this continuing education activity for 1.0 contact hour(s) (0.05 CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number - JA4008073-9999-24-098-H01-P)
Type of Activity: Knowledge
For Pharmacists: Upon successfully completing the post-test with a score of 80% or better and the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
- During the period 5/2/2024 through 5/2/2026 participants must read the learning objectives and faculty disclosures and study the educational activity.
- Once the participant has passed the post-test with a score of 80% or higher (unlimited attempts allowed), and the course evaluation has been completed, credit will be automatically requested to the CRO the participant has selected during the registration process.
- Certificates will be available to the participant for printing after passing the post-test and completing the course evaluation.
Satisfactory Completion
Learners must listen to each self-directed audio recording while following along with the visual slides/read the articles, pass the post-test with a score of 80% or higher (unlimited attempts) and complete an evaluation form to receive a certificate of completion. You must participate in the entire activity as partial credit is not available. If you are seeking continuing education credit for a specialty not listed below, it is your responsibility to contact your licensing/certification board to determine course eligibility for your licensing/certification requirement.
| | Original Course Date: March 01, 2021
On-Demand Release Date: Available Now |
Approved Credit: ACCME (MD/DO): 1 hour AMA PRA Category 1 Credit(s)™ACCME (non-MD/DO): 1 hour AMA PRA Category 1 Credit(s)™CoP: 0.00 hours Certificate of Participation: 1 hour AMA PRA Category 1 Credit(s)™ / ABIM Maintenance of Certification Part 2 CreditACPE: 1 hour Contact HourANCC: 1 hour Contact Hour
| MORE INFO |
|
Clinician Courses by Cancer Type
|
|





































































































.png)


.png)






 In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by the Postgraduate Institute for Medicine and Society for Immunotherapy of Cancer. Postgraduate Institute for Medicine is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.

 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Society for Immunotherapy of Cancer (SITC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team. Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.5 MOC points in the American Board of Internal Medicine’s (ABIM) Maintenance of Certification (MOC) program. It is the CME activity provider’s responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.


 As diagnostic and therapeutic advances intersect, there is a significant impact on oncology patient care and increased the use of molecular testing to guide treatment decision-making. While these practices are evidence-based, complex, and incorporated into clinical guidelines, their adoption is fraught with challenges including the multiple companion diagnostic tests associated with immunotherapies. Ask an expert pathologist and oncologist your burning questions as they discuss their strategies for improving molecular testing, which biomarkers to test for, interpreting molecular test results, and how that translates to selecting immunotherapy for patients with solid tumors.
As diagnostic and therapeutic advances intersect, there is a significant impact on oncology patient care and increased the use of molecular testing to guide treatment decision-making. While these practices are evidence-based, complex, and incorporated into clinical guidelines, their adoption is fraught with challenges including the multiple companion diagnostic tests associated with immunotherapies. Ask an expert pathologist and oncologist your burning questions as they discuss their strategies for improving molecular testing, which biomarkers to test for, interpreting molecular test results, and how that translates to selecting immunotherapy for patients with solid tumors.







 Experts discuss the evolving role of checkpoint inhibition in patients with advanced bladder cancer.
Experts discuss the evolving role of checkpoint inhibition in patients with advanced bladder cancer. Nursing experts discuss the emergence of immunotherapy in GI cancers, focusing on the role of biomarkers and the management of irAEs.
Nursing experts discuss the emergence of immunotherapy in GI cancers, focusing on the role of biomarkers and the management of irAEs. Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules).
Exemplar video presentations from the live Advances in Cancer Immunotherapy™ (ACI) programs are available as an online Video Series course. One video has been selected for each topic of the 2019 ACI program (eight total modules). Apply best practices to monitoring for, diagnose, and manage potential irAEs in patient with cancer receiving ICIs
Apply best practices to monitoring for, diagnose, and manage potential irAEs in patient with cancer receiving ICIs












